Abstract
Background
Methods
Results
Conclusion
References
Table 1
Study population and associated demographics
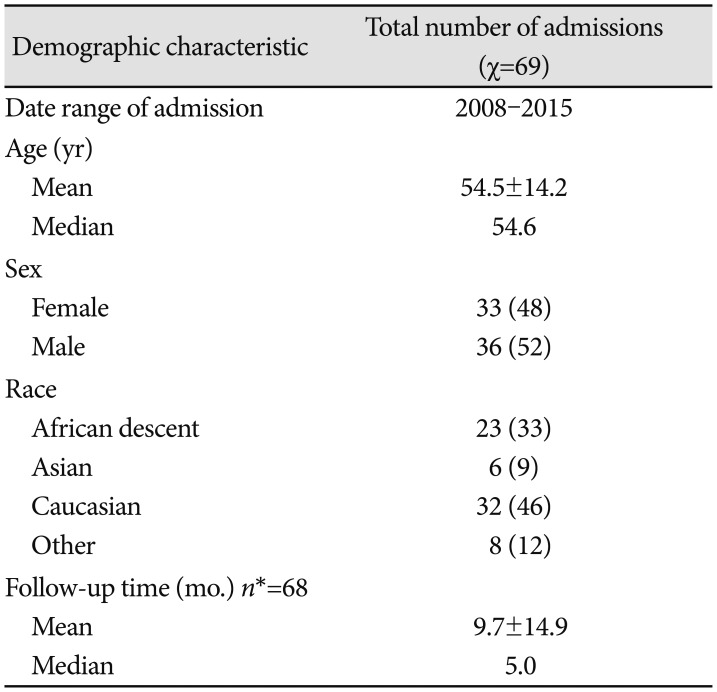
Table 2
Clinical presentations of pituitary adenoma patients
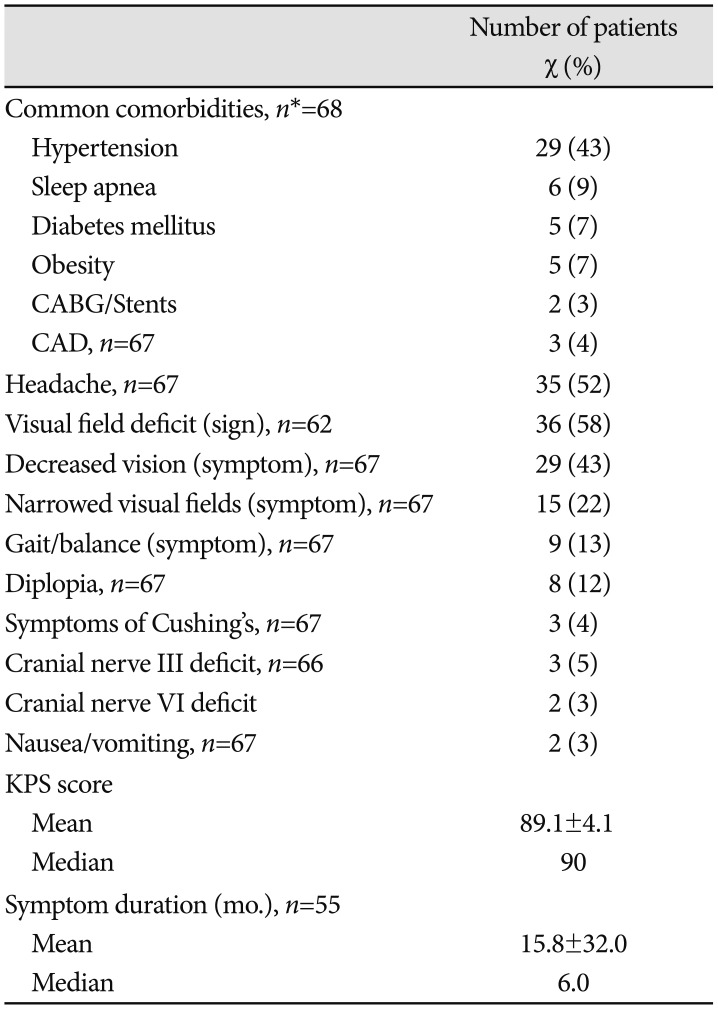
Data are expressed as number (percentage) of admissions presenting with each variable or mean±SD. Percentages have been rounded and may not add up to 100. *n indicates the number of admissions with available data. If no n is specified, all admissions were included. CABG, coronary artery bypass grafting; CAD, coronary artery disease; KPS, Karnofsky Performance Status
Table 3
Pituitary adenoma radiological, surgical, and laboratory results
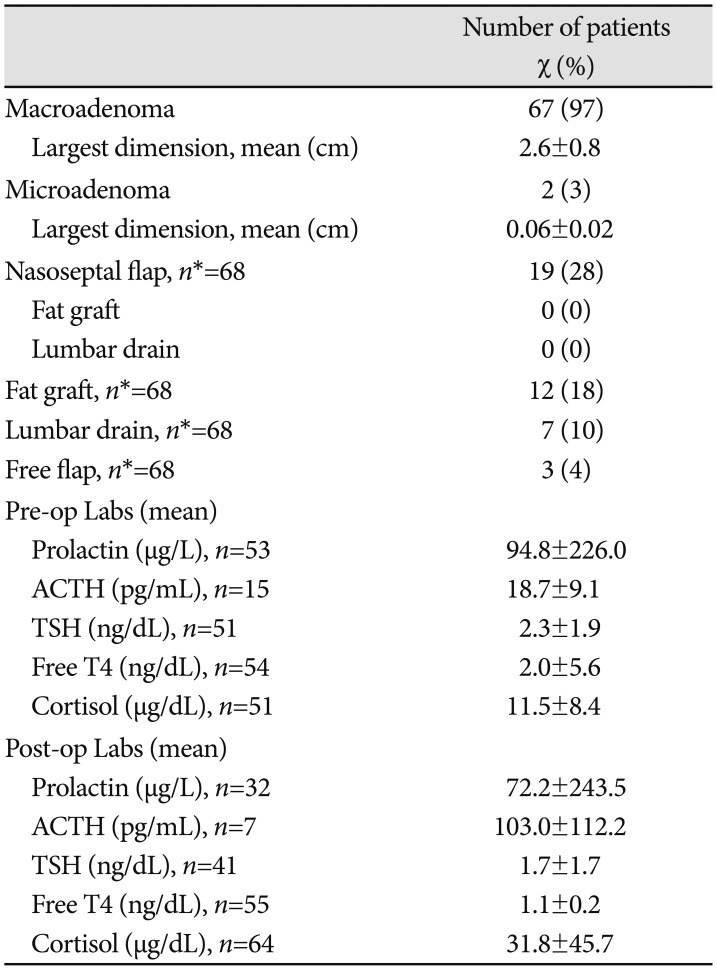
Data are expressed as number (percentage) of admissions presenting with each variable or mean±SD. Percentages have been rounded and may not add up to 100. *n indicates the number of admissions with available data. If no n is specified, all admissions were included. ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; T4, thyroxine 4
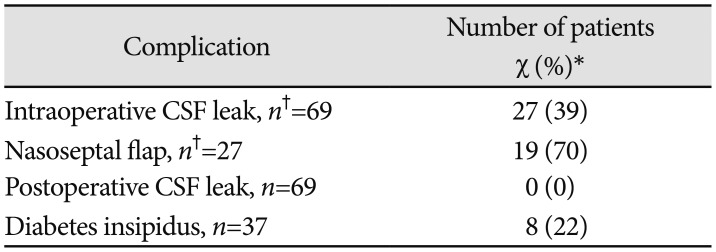
Table 4
Perioperative and postoperative complications
| Complication | Number of patients χ (%)* |
|---|---|
| Intraoperative CSF leak, n†=69 | 27 (39) |
| Nasoseptal flap, n†=27 | 19 (70) |
| Postoperative CSF leak, n=69 | 0 (0) |
| Diabetes insipidus, n=37 | 8 (22) |
*Unless otherwise noted, data are expressed as number (percentage) of admissions presenting with each variable. Percentages have been rounded and may not add up to 100, †n indicates the number of admissions with available data. If no n is specified, all admissions were included. CSF, cerebrospinal fluid
Table 5
Summary of the literature
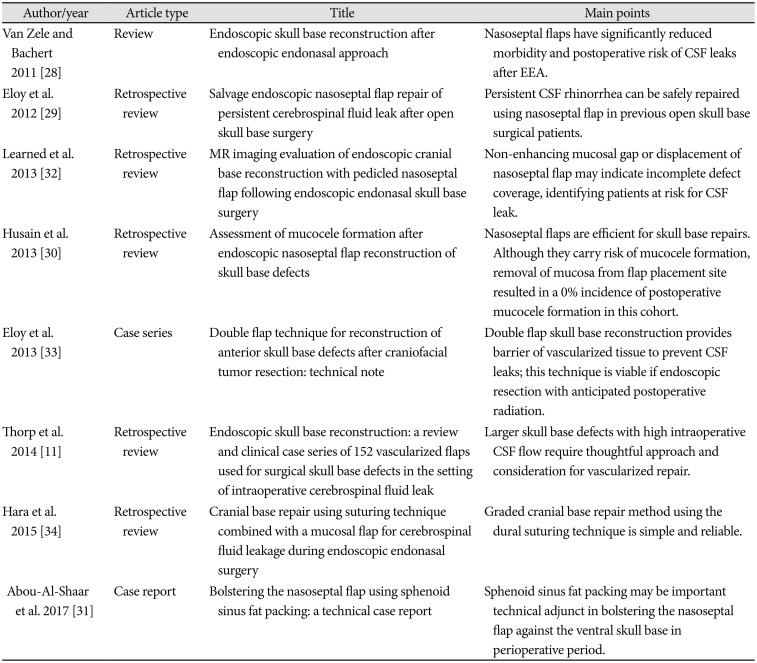
| Author/year | Article type | Title | Main points |
|---|---|---|---|
| Van Zele and Bachert 2011 [28] | Review | Endoscopic skull base reconstruction after endoscopic endonasal approach | Nasoseptal flaps have significantly reduced morbidity and postoperative risk of CSF leaks after EEA. |
| Eloy et al. 2012 [29] | Retrospective review | Salvage endoscopic nasoseptal flap repair of persistent cerebrospinal fluid leak after open skull base surgery | Persistent CSF rhinorrhea can be safely repaired using nasoseptal flap in previous open skull base surgical patients. |
| Learned et al. 2013 [32] | Retrospective review | MR imaging evaluation of endoscopic cranial base reconstruction with pedicled nasoseptal flap following endoscopic endonasal skull base surgery | Non-enhancing mucosal gap or displacement of nasoseptal flap may indicate incomplete defect coverage, identifying patients at risk for CSF leak. |
| Husain et al. 2013 [30] | Retrospective review | Assessment of mucocele formation after endoscopic nasoseptal flap reconstruction of skull base defects | Nasoseptal flaps are efficient for skull base repairs. Although they carry risk of mucocele formation, removal of mucosa from flap placement site resulted in a 0% incidence of postoperative mucocele formation in this cohort. |
| Eloy et al. 2013 [33] | Case series | Double flap technique for reconstruction of anterior skull base defects after craniofacial tumor resection: technical note | Double flap skull base reconstruction provides barrier of vascularized tissue to prevent CSF leaks; this technique is viable if endoscopic resection with anticipated postoperative radiation. |
| Thorp et al. 2014 [11] | Retrospective review | Endoscopic skull base reconstruction: a review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak | Larger skull base defects with high intraoperative CSF flow require thoughtful approach and consideration for vascularized repair. |
| Hara et al. 2015 [34] | Retrospective review | Cranial base repair using suturing technique combined with a mucosal flap for cerebrospinal fluid leakage during endoscopic endonasal surgery | Graded cranial base repair method using the dural suturing technique is simple and reliable. |
| Abou-Al-Shaar et al. 2017 [31] | Case report | Bolstering the nasoseptal flap using sphenoid sinus fat packing: a technical case report | Sphenoid sinus fat packing may be important technical adjunct in bolstering the nasoseptal flap against the ventral skull base in perioperative period. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download