Abstract
Purpose
A dedifferentiated chondrosarcoma is a rare lethal tumor characterized by a low grade chondrosarcoma juxtaposed with a high grade dedifferentiated sarcoma, such as osteosarcoma, fibrosarcoma. The aim of our study was to document the clinical manifestation and oncologic outcomes of a dedifferentiated chondrosarcoma.
Materials and Methods
This study identified 11 patients who were diagnosed and treated for dedifferentiated chondrosarcoma between January 2007 and December 2016. The identified cohort was then reviewed regarding age, sex, symptom onset, tumor location, magnetic resonance imagings (MRIs), surgical margin, and pathologic diagnosis. The time to local recurrence and/or metastasis, follow-up duration, and the patients' final status were analyzed.
Results
The patients were comprised of 7 males and 4 females with a mean age of 54 years (range, 33–80 years). The location of the tumor was in the femur in 6 cases, pelvis in 4 cases, and metatarsal in 1 case. The average tumor diameter was 12.7 cm (range, 6.0–26.1 cm). At the time of diagnosis, 2 patients showed pathologic fracture; 1 patient was Enecking stage IIA, 9 patients were stage IIB, and 1 patient was stage III. Eight patients were classified as a primary dedifferentiated chondrosarcoma and 3 patients were secondary. One of the primary lesions was misinterpreted initially as a low grade chondroid lesion by MRI and underwent curettage. Local recurrence occurred in 8 cases and distant metastasis occurred in 10 cases with a mean duration of 8 months (range, 2–23 months) and 7 months (range, 1–32 months), respectively. The three-year overall survival of patients with dedifferentiated chondrosarcoma was 18%, and 10 patients died due to disease progression.
Figures and Tables
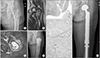 | Figure 1An 80-year-old female with left hip pain that developed 2 months ago (case 5). (A) The initial plain radiograph shows well marginated calcified bone lesion at the proximal femur. Note the oblique fracture line with minimal displacement. The image shows no endosteal scalloping. (B, C) The coronal T2 and axial short tau inversion recovery T2-weighted magnetic resonance imagings show a lobular marginated bone lesion with peripheral soft tissue edema. Subtle cortical disruption can be seen at the greater trochanter. The initial presumptive diagnosis based on the images was low grade chondrosarcoma. (D) Curettage and bone cementing was performed. (E) The specimen was given a diagnosis of dedifferentiated chondrosarcoma. Low-power histologic photomicrograph shows the cartilage component and dedifferentiated component (H&E, ×100). (F) Cartilaginous areas show mild nuclear pleomorphism and hyperchromatism (H&E, ×400). (G) The dedifferentiated part consists of osteosarcomatous tumor cells without any chondroblastic differentiation (H&E, ×400). (H) Wide excision and reconstruction using a tumor prosthesis was performed. |
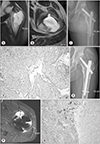 | Figure 2A 46-year-old male with left hip pain that developed 6 months ago (case 10). (A) The initial coronal T2 weighted magnetic resonance imaging (MRI) shows an intraosseous well-marginated bone lesion. (B) The initial axial T2 weighted MRIs shows a subtle cortical disruption with soft tissue involvement. (C) Curettage and bone grafting were performed at the referral hospital. The diagnosis was chondrosarcoma. (D) The specimen revealed a histologic grade 2 chondrosarcoma (H&E, ×200). (E) Left hip pain re-developed 3 months after surgery. Note the soft tissue mass at the gluteal muscle and endosteal scalloping of the femur. (F) Axial T2 weighted MR image shows the cortical destruction with soft tissue mass formation. Wide excision and reconstruction was performed under the impression of recurrence. (G) This photomicrograph shows the bimorphic pattern of grade 2 chondrosarcoma and osteoblastic osteosarcoma (H&E, ×200). |
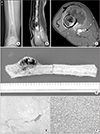 | Figure 3A 60-year-old man with right thigh mass that developed 2 months ago (case 8). (A) The initial plain radiograph shows chondroid calcification with endosteal scalloping. (B, C) The sagittal T2 and axial enhanced T1 weighted magnetic resonance imagings show cortical disruption and the presence of a soft tissue mass. (D) An intercalary resection was performed at Korea Cancer Center Hospital. Note the anterior soft tissue mass with cortical disruption. (E) This high-power histologic photomicrograph shows the grade 1 chondrosarcoma component (H&E, ×400). (F) The anaplastic component showed a high-grade sarcoma in which the transformation origin was difficult to specify (H&E, ×400). |
References
2. Frassica FJ, Unni KK, Beabout JW, Sim FH. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J Bone Joint Surg Am. 1986; 68:1197–1205.


3. Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007; 43:2060–2065.


4. Liu C, Xi Y, Li M, et al. Dedifferentiated chondrosarcoma: radiological features, prognostic factors and survival statistics in 23 patients. PLoS One. 2017; 12:e0173665.

5. Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006; 106:2682–2691.


6. Hwang PG, Won JK, Kim MA, Kim HS, Lee SH, Kim CJ. Dedifferentiated chondrosarcoma with giant cell-rich sarcomatous component resembling giant cell tumor: a case report. Korean J Pathol. 2004; 38:345–349.
7. Park JH, Koh HS, Lee SY. Dedifferentiated chondrosarcoma from low grade chondrosarcoma. J Korean Bone Joint Tumor Soc. 2005; 11:213–218.
8. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980; (153):106–120.

9. Mercuri M, Picci P, Campanacci L, Rulli E. Dedifferentiated chondrosarcoma. Skeletal Radiol. 1995; 24:409–416.


10. MacSweeney F, Darby A, Saifuddin A. Dedifferentiated chondrosarcoma of the appendicular skeleton: MRI-pathological correlation. Skeletal Radiol. 2003; 32:671–678.


11. Bovée JV, Cleton-Jansen AM, Rosenberg C, Taminiau AH, Cornelisse CJ, Hogendoorn PC. Molecular genetic characterization of both components of a dedifferentiated chondrosarcoma, with implications for its histogenesis. J Pathol. 1999; 189:454–462.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download