1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810. PMID:
26903338.
2. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22:707–710. PMID:
8844239.
3. Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998; 26:1793–1800. PMID:
9824069.
4. Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999; 25:686–696. PMID:
10470572.
5. Fan SL, Miller NS, Lee J, Remick DG. Diagnosing sepsis-The role of laboratory medicine. Clin Chim Acta. 2016; 460:203–210. PMID:
27387712.
6. Chan D, Ng L. Novel biomarkers in heart failure: adrenomedullin and proenkephalin Cardiac biomarkers. In : Maisel AS, Jaffe AS, editors. Case studies and clinical correlations. Springer International Publishing;2016. p. 285–296.
7. Hamid SA, Baxter GF. Adrenomedullin: regulator of systemic and cardiac homeostasis in acute myocardial infarction. Pharmacol Ther. 2005; 105:95–112. PMID:
15670621.
9. Chen S, Lu X, Zhao Q, Wang L, Li H, Huang J. Association of adrenomedullin gene polymorphisms and blood pressure in a Chinese population. Hypertens Res. 2013; 36:74–78. PMID:
22932875.
10. Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000; 101:498–503. PMID:
10662746.
11. Eto T, Kato J, Kitamura K. Regulation of production and secretion of adrenomedullin in the cardiovascular system. Regul Pept. 2003; 112:61–69. PMID:
12667626.
12. Geven C, Kox M, Pickkers P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front Immunol. 2018; 9:292. PMID:
29520277.
13. Ter Maaten JM, Kremer D, Demissei BG, Struck J, Bergmann A, Anker SD, et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur J Heart Fail. 2019; 3. 06. DOI:
10.1002/ejhf.1437. [Epub ahead of print].
14. Voors AA, Kremer D, Geven C, Ter Maaten JM, Struck J, Bergmann A, et al. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail. 2019; 21:163–171. PMID:
30592365.
15. Weber J, Sachse J, Bergmann S, Sparwaßer A, Struck J, Bergmann A. Sandwich immunoassay for bioactive plasma adrenomedullin. J Appl Lab Med. 2017; 2:222–233.
16. Caironi P, Latini R, Struck J, Hartmann O, Bergmann A, Maggio G, et al. Circulating biologically active adrenomedullin (bio-ADM) predicts hemodynamic support requirement and mortality during sepsis. Chest. 2017; 152:312–320. PMID:
28411114.
17. Mebazaa A, Geven C, Hollinger A, Wittebole X, Chousterman BG, Blet A, et al. Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) study. Crit Care. 2018; 22:354. PMID:
30583748.
18. Geven C, Blet A, Kox M, Hartmann O, Scigalla P, Zimmermann J, et al. A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2). BMJ Open. 2019; 9:e024475.
19. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017; 45:486–552. PMID:
28098591.
20. Williams JM, Keijzers G, Macdonald SP, Shetty A, Fraser JF. Review article: sepsis in the emergency department-Part 3: treatment. Emerg Med Australas. 2018; 30:144–151. PMID:
29569847.
22. Simon TP, Martin L, Doemming S, Humbs A, Bruells C, Kopp R, et al. Plasma adrenomedullin in critically ill patients with sepsis after major surgery: A pilot study. J Crit Care. 2017; 38:68–72. PMID:
27865148.
23. Marino R, Struck J, Maisel AS, Magrini L, Bergmann A, Di Somma S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit Care. 2014; 18:R34. PMID:
24533868.
24. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016; 12:610–623. PMID:
27573728.
25. Martin L, Derwall M, Al Zoubi S, Zechendorf E, Reuter DA, Thiemermann C, et al. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest. 2019; 155:427–437. PMID:
30171861.
26. Yang HS, Hur M, Kim H, Magrini L, Marino R, Di Somma S, et al. Soluble suppression of tumorigenicity 2 and echocardiography in sepsis. Ann Lab Med. 2016; 36:590–594. PMID:
27578513.
27. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18:891–975. PMID:
27207191.
28. Kremer D, Ter Maaten JM, Voors AA. Bio-adrenomedullin as a potential quick, reliable, and objective marker of congestion in heart failure. Eur J Heart Fail. 2018; 20:1363–1365. PMID:
29932477.
29. Self WH, Storrow AB, Hartmann O, Barrett TW, Fermann GJ, Maisel AS, et al. Plasma bioactive adrenomedullin as a prognostic biomarker in acute heart failure. Am J Emerg Med. 2016; 34:257–262. PMID:
26577429.
30. Tolppanen H, Rivas-Lasarte M, Lassus J, Sans-Roselló J, Hartmann O, Lindholm M, et al. Adrenomedullin: a marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Ann Intensive Care. 2017; 7:6. PMID:
28050899.
31. Meeran K, O'Shea D, Upton PD, Small CJ, Ghatei MA, Byfield PH, et al. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab. 1997; 82:95–100. PMID:
8989240.
32. Mallamaci F, Zoccali C, Parlongo S, Cutrupi S, Tripepi G, Postorino M. Plasma adrenomedullin during acute changes in intravascular volume in hemodialysis patients. Kidney Int. 1998; 54:1697–1703. PMID:
9844147.
33. Valenzuela-Sánchez F, Valenzuela-Méndez B, Rodríguez-Gutiérrez JF, Estella-García Á, González-García MÁ. New role of biomarkers: mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann Transl Med. 2016; 4:329. PMID:
27713887.
34. Elke G, Bloos F, Wilson DC, Brunkhorst FM, Briegel J, Reinhart K, et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis - a secondary analysis of a large randomised controlled trial. Crit Care. 2018; 22:79. PMID:
29562917.
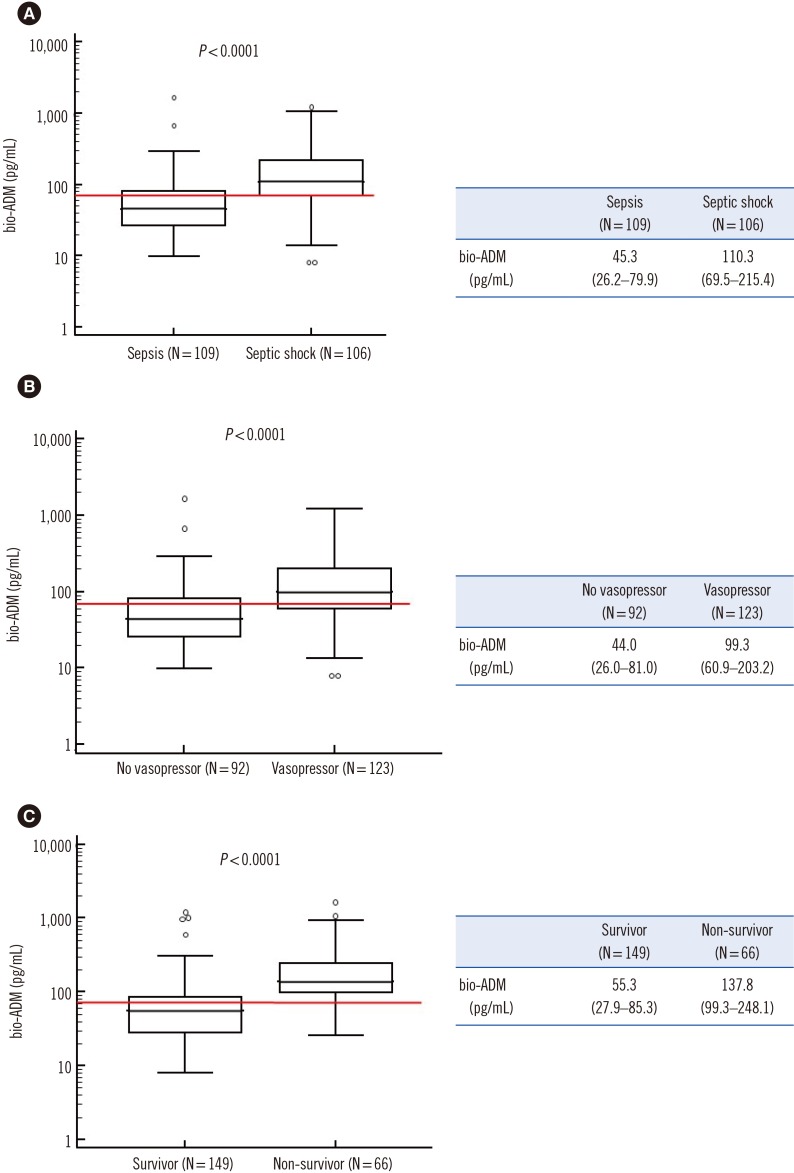

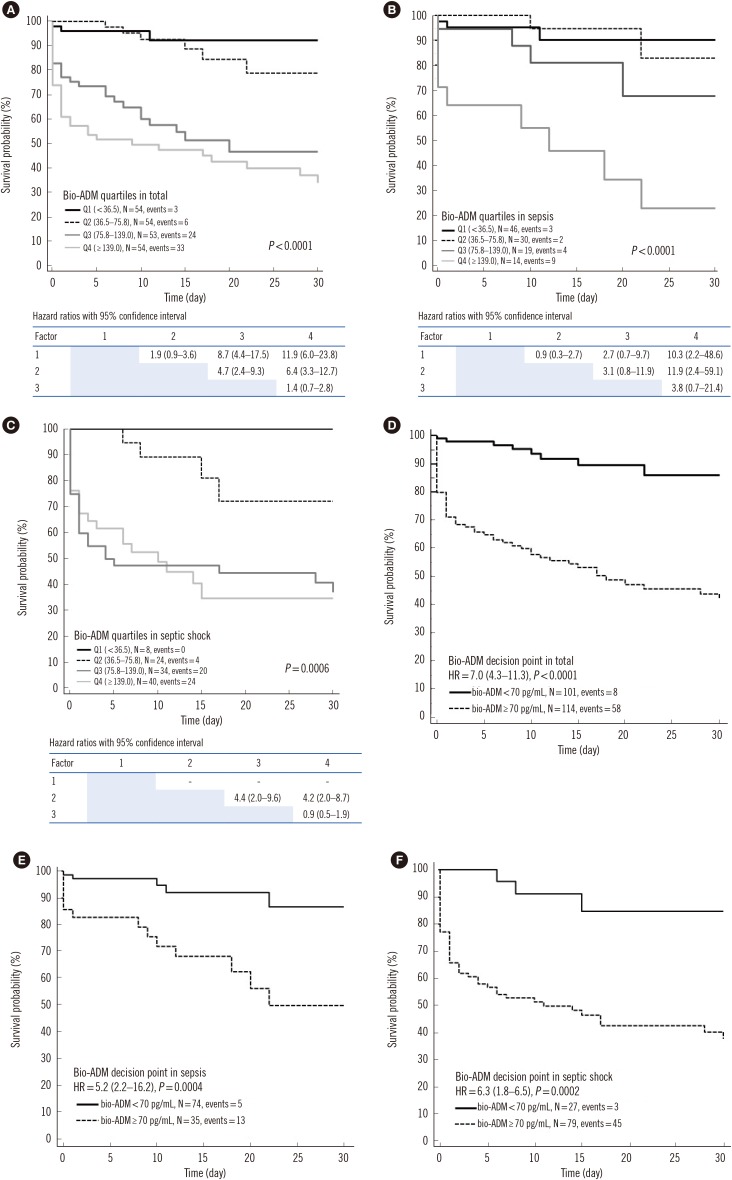
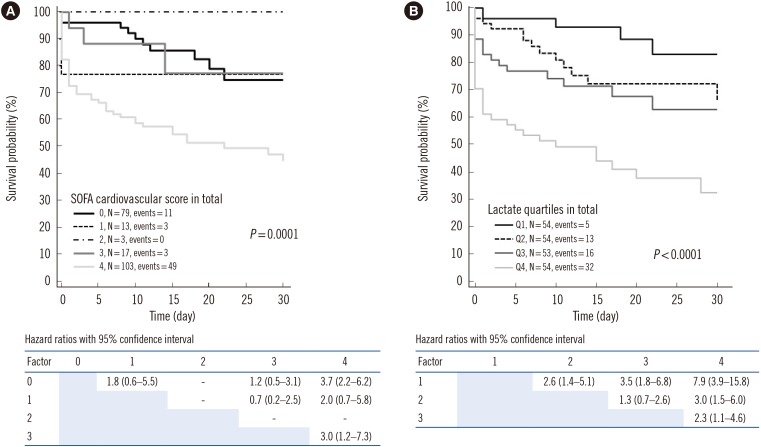
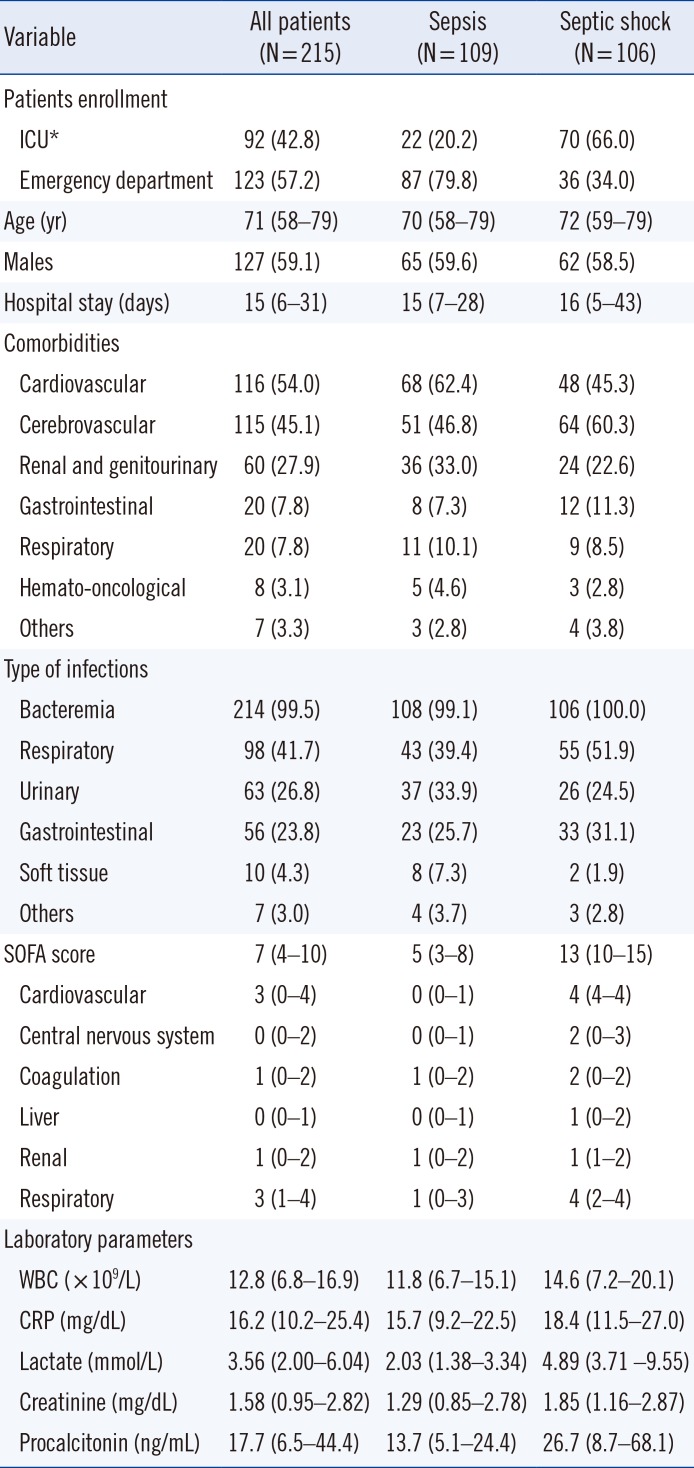




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download