INTRODUCTION
Mass spectrometry (MS) represents a key technology in biomedical research areas, such as proteomics, pharmacology, and metabolomics [
1]. With the advent of soft ionization techniques, such as electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI), the use of liquid chromatography-tandem MS (LC-MS/MS) has substantially increased in clinical laboratories [
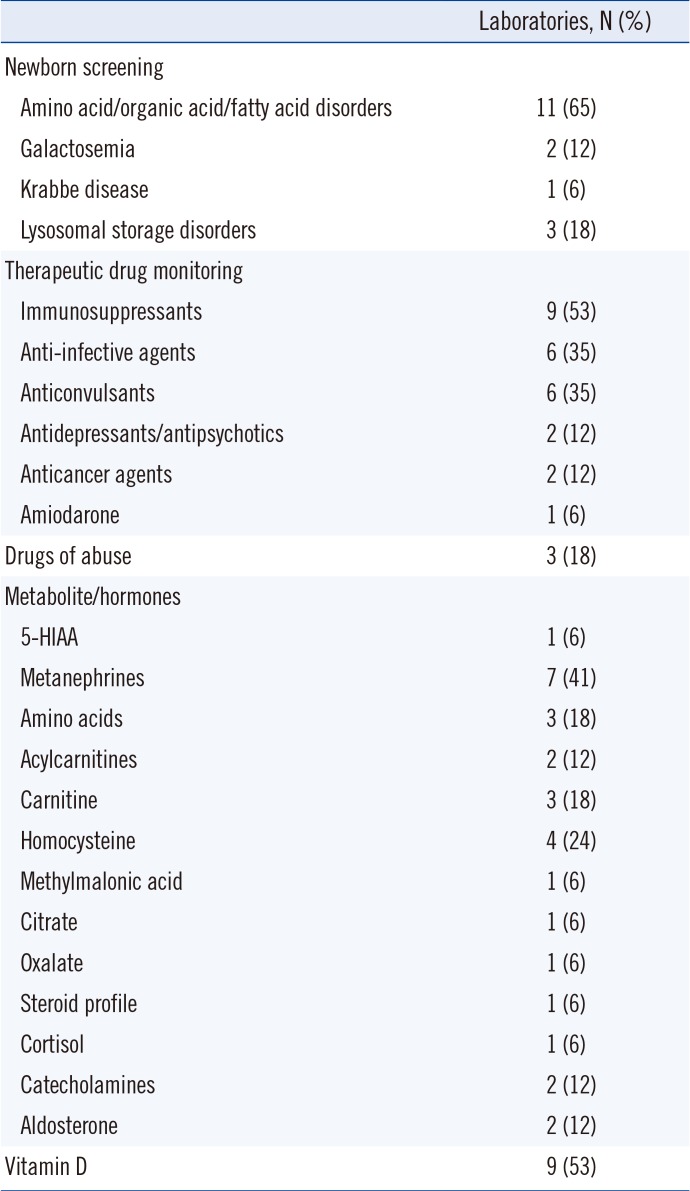
23]. Current clinical applications of LC-MS/MS include screening for inherited metabolic disorders, measurement of numerous small-molecule biomarkers, and quantification of drugs and their metabolites [
1]. The strengths of LC-MS/MS are its high selectivity and sensitivity, capability for multi-analyte analyses, and high throughput.
As an effort to popularize and improve the use of LC-MS/MS in clinical laboratories across Korea, a questionnaire survey was conducted by the Clinical Mass Spectrometry Research Committee (CMSRC) of the Korean Society of Clinical Chemistry (KSCC). It aimed to provide an accurate and updated overview of clinical LC-MS/MS testing in Korean clinical laboratories and shed light on the challenges of using LC-MS/MS in this setting.
Go to :

METHODS
Survey population
The survey population consisted of all 19 clinical laboratories performing clinical LC-MS/MS tests in medical institutions (hospitals and medical centers) and referral medical laboratories accredited by the Korean Laboratory Accreditation Program (KOLAS) [
4]. The survey was performed from April to May 2018. The questionnaire was e-mailed to the directors and laboratory physicians in charge of these 19 laboratories. This study was approved by the Institutional Review Board (IRB)/Ethics Committee of Seoul St. Mary's Hospital, Korea (IRB No. KC18QCDI0566).
Questionnaire
The questionnaire was in Korean, with multiple-choice questions, and consisted of two sections. The first comprised questions on the general characteristics of the LC-MS/MS laboratory: type of medical institution; number of LC-MS/MS instruments; number of LC-MS/MS laboratory physicians, technicians, and researchers; years of experience with clinical LC-MS/MS tests; clinical LC-MS/MS tests performed; laboratory area; and instrument management (nitrogen gas supply and preventive maintenance plans).
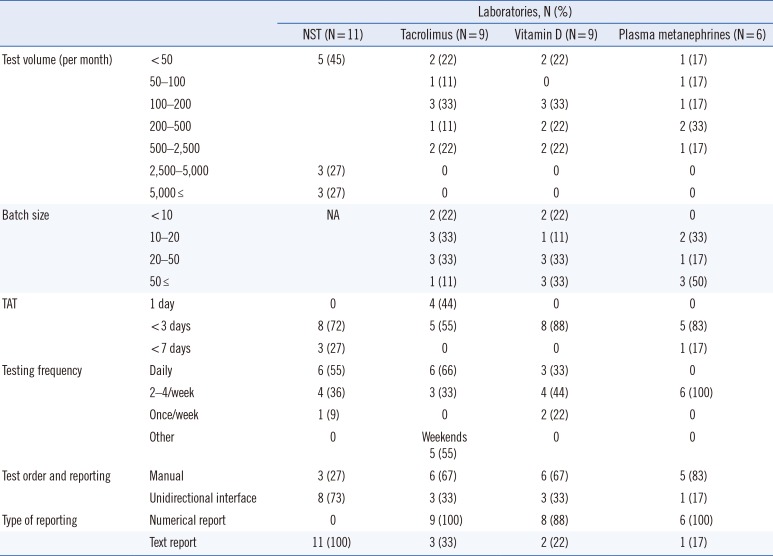
The second section comprised questions on four commonly utilized clinical diagnostic tests: newborn screening test (NST), tacrolimus test, vitamin D test, and plasma metanephrine test. The questionnaire asked about sample pretreatment and sample volume, test volume, turnaround time (TAT), testing frequency, calibrators, internal standards, reagents, internal quality control, test reporting, proficiency testing, and components of method validation.
Data analysis
An Excel spreadsheet was used to summarize the data. Categorical data were summarized as frequency and percentage, and continuous data were summarized as median and range. Normality was assessed using the D'Agostino and Pearson normality test. The association between the types of the clinical laboratory and TAT or test volume was evaluated using Fisher's exact tests. The correlation between the number of LC-MS/MS instruments and the number of laboratory medicine physicians, the number of laboratory technicians, and the number of clinical LC-MS/MS tests was assessed using Pearson's correlation coefficient. GraphPad Prism 7.05 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses. P<0.05 was considered statistically significant.
Go to :

DISCUSSION
This survey summarizes the current practices and characteristics of clinical LC-MS/MS laboratories in Korea. It shows that LC-MS/MS has been successfully introduced in many clinical laboratories, with major applications in newborn screening, therapeutic drug monitoring, endocrinology, and nutritional assessment. The expansion and integration of LC-MS/MS into clinical laboratories are reflected in its proportion in EQA programs and in the fact that 7% and 2% of all participating laboratories in the 2018 KEQAS EQA scheme use LC-MS/MS for tacrolimus and vitamin D testing, respectively.
However, the number of clinical LC-MS/MS laboratories in Korea (19) is still small, only 6% of the total number of clinical laboratories accredited by the KOLAS (305). Further, clinical LC-MS/MS laboratories are limited to university hospitals (65%) and large referral medical laboratories (35%). The proportion of LC-MS/MS in Korean EQA programs is lower than that in global EQA programs (17% and 10% of all participating laboratories for tacrolimus and vitamin D tests, respectively) [
5]. Therefore, considering the global growth of clinical LC-MS/MS tests, there is still room for further expansion of LC-MS/MS in Korea.
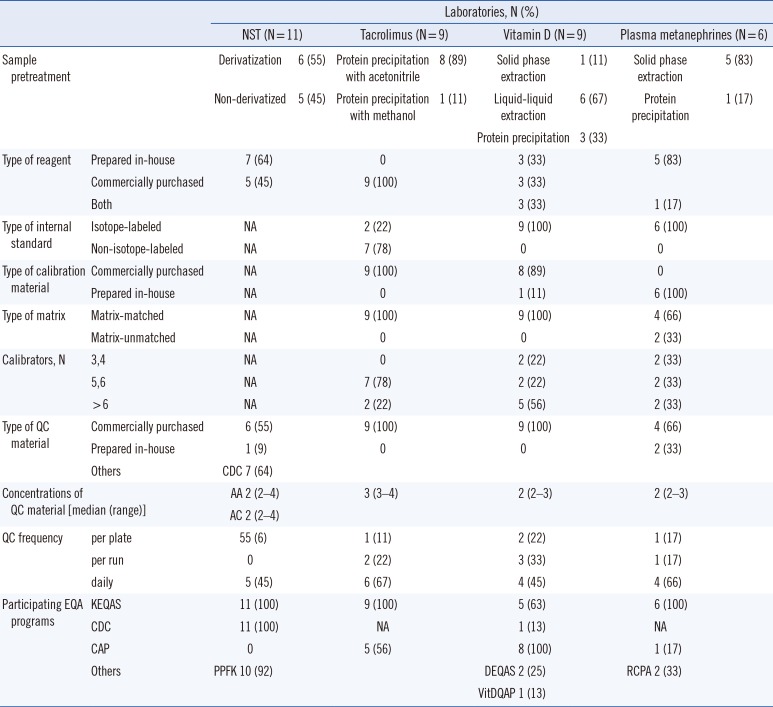
The original sample preparation methods for the LC-MS/MS NST used butyl esterification (derivatization). However, with the improved sensitivity of MS instruments, it is possible to detect amino acids and acylcarnitines as their native free acids (non-derivatized). We found that the proportion of derivatization and non-derivatized sample preparation methods used was similar, in accordance with recent CDC NST QC data summaries [
6]. Protein precipitation is the most commonly used method for extracting immunosuppressants because it is simple, cheap, and less time-consuming [
7]. For the vitamin D tests, 67% and 33% of all laboratories used liquid-liquid extraction and protein precipitation methods, respectively, similar to the proportions reported in the vitamin D EQA Scheme (DEQAS) data [
8]. To analyze plasma metanephrines, solid phase extraction has been the sample preparation method of choice [
9]. In the present survey, all but one laboratory used solid phase extraction for sample preparation.
The addition of an internal standard to samples before analysis represents the single most valuable method enhancement that MS can offer [
10]. All responding laboratories used methods that included the use of an internal standard. The majority of laboratories used a stable isotope-labeled form of the measured analyte. However, for tacrolimus, a non-isotopic internal standard, ascomycin, was used by the majority of laboratories. Although a recent study did not observe any differences between the use of ascomycin and an isotope-labeled internal standard, tacrolimus-
13C,D
2 [
11], other studies reported better results using tacrolimus-
13C,D
2 than ascomycin [
12]. With the recent availability of commercial isotope-labeled internal standards for immunosuppressants (e.g., Chromsystems, Munich, Germany, or Recipe, Munich, Germany), the use of isotope-labeled internal standards in immunosuppressant LC-MS/MS tests is likely to increase in the near future.
Regarding quality assurance and QC for LC-MS/MS, the CLSI document C62-A suggests that a minimum of two concentrations of QC materials should be measured at least every 24 hours during patient testing [
10]. We found that at least two concentrations of QC materials were measured for each LC-MS/MS test, and all laboratories were using QC acceptability criteria based on their own established QC means and SDs derived from repetitive analysis of control materials. All laboratories reported participation in one or more EQA programs. As EQA is one of the most preferred methods for assessing test accuracy, these laboratories reported using LC-MS/MS within a quality framework that addresses both internal and external quality assurance.
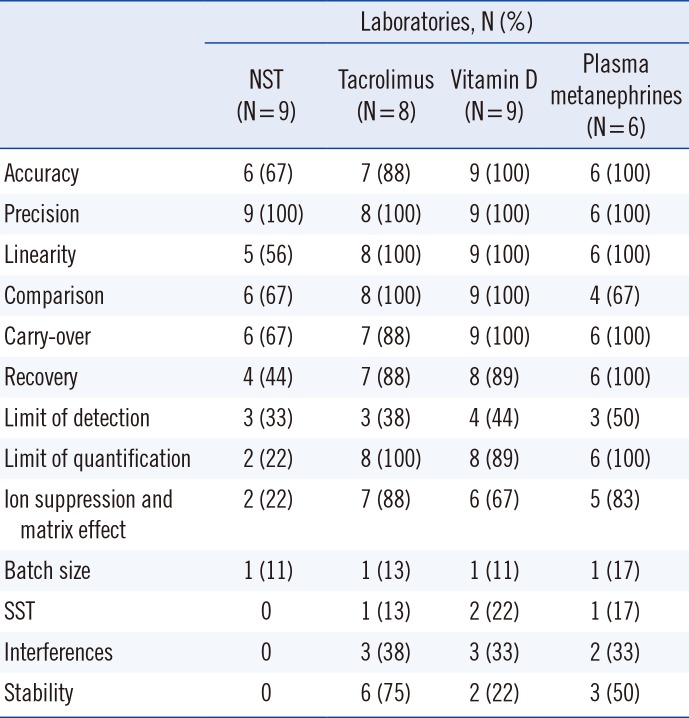
One of the potential obstacles preventing the adoption of LC-MS/MS in routine clinical laboratories is the seemingly overwhelming method validation requirements required to meet the guidelines for clinical applications. Although there are multiple guidelines for the validation of bioanalytical methods [
131415], including the CLSI C62-A [
10], regulatory requirements differ somewhat across countries and laboratory accreditation agencies. In this regard, the development of regional/national guidelines for LC-MS/MS validation for clinical laboratories that address regional regulations could facilitate the use of LC-MS/MS in medium-sized community hospitals and regional clinical laboratories in Korea.
This study has some limitations. Because this survey was an initiative of the CMSRC of the KSCC, it focused primarily on LC-MS/MS testing in clinical chemistry applications. Thus, other areas of clinical MS, such as gas chromatography-MS, inductively coupled plasma MS, and matrix-assisted laser desorption ionization time-of-flight MS (MALDI-TOF MS), in clinical microbiology were not assessed. Another limitation was the fact that the number of responding laboratories was relatively low, making it difficult to draw definitive conclusions.
However, this survey is the first to describe various aspects of LC-MS/MS in clinical laboratories in Korea. Furthermore, it is important to understand the current status of clinical LC-MS/MS laboratory practices to form a basis upon which future harmonization and advancements can be made. Further studies attempting to harmonize and enhance the analytical robustness of LC-MS methods on a nationwide basis will be necessary.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download