INTRODUCTION
PROTOCOL DEVELOPMENT AND FOCUSED QUESTION
Eligibility criteria
• Population: patients older than 18 diagnosed and treated for periodontitis under SPT.
• Intervention: surgical or non-surgical periodontal therapy.
• Comparison: surgical versus non-surgical therapy, different modalities of surgical and non-surgical therapy in controlled studies.
• Outcome: disease progression (defined as CAL loss).
• Study design: randomized controlled trials (RCTs), prospective cohort studies and prospective case series with a minimum of 10 patients (5 per group in controlled studies).
Exclusion criteria
• Review or preclinical studies
• Studies with less than 5 years of follow-up
• Retrospective studies
• Studies aiming at regenerating the periodontum
• Studies with unspecified or unstandardized treatments
• Studies reporting on specific populations, such as patients with diabetes
• Studies reporting on early-onset periodontitis or refractory periodontitis
INFORMATION SOURCES AND SEARCH
Electronic search
Manual search
Search strategy
(scaling and root planing) OR (basic periodontal therapy) OR (non surgical periodontal therapy)
(periodontal access flap surgery) OR (open flap debridement) OR (osseous resective surgery) OR (modified widman flap) OR (periodontal access surgery)
(#1) OR (#2)
(periodontal disease progression) OR (disease progression) [mh] OR (clinical attachment level) OR (periodontal attachment loss) [mh] OR (treatment outcomes) [mh] OR (longitudinal studies) [mh]
(#3) AND (#4)
(periodontal regeneration) OR (peri-implantitis) [mh]
(#5) NOT (#6)
Screening methods
Data extraction
Quality assessment (risk of bias in individual publications)
Data analyses
RESULTS
Search and publication characteristics
Quality assessment of the included publications
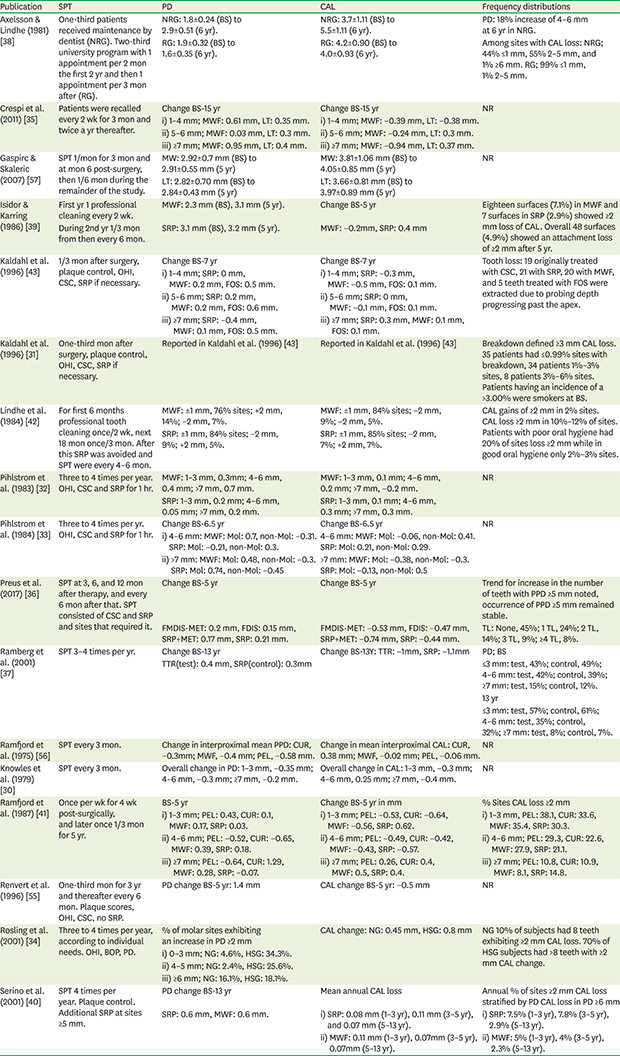
Table 1
Risk of bias of randomized studies according to the Cochrane Collaboration recommendations (Higgins and Green, 2011)

| References | Selection bias | Performance bias | Detection bias | Attrition bias | Selective reporting bias | Other potential risk of bias | |
|---|---|---|---|---|---|---|---|
| Sequence generation | Allocation concealment | ||||||
| Crespi et al. (2011) [35] | High | High | High | High | High | Low | Low |
| Gaspirc & Skaleric (2007) [57] | High | High | High | High | Low | High | Low |
| Kaldahl et al. (1996) [43] | High | High | High | High | High | High | High |
| Lindhe et al. (1984) [42] | High | High | High | High | High | Low | Low |
| Pihlstrom et al. (1983) [32] | High | High | High | High | High | Low | High |
| Preus et al. (2017) [36] | Low | Low | High | High | Low | High | Low |
| Ramfjord et al. (1975) [56] | High | High | High | High | Low | Low | Low |
| Knowles et al. (1979) [30] | High | High | High | High | Unclear | High | High |
| Ramfjord et al. (1987) [41] | High | High | High | High | High | Low | Low |
| Serino et al. (2001) [40] | High | High | High | High | High | Low | Low |
Description of the included publications
Interventions
Table 2
Methodological characteristics and outcomes measured by the included studies

| Authors (year) | Reference | Intervention | Design | FU (yr) | Patients | Test | Control | Other treatments | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Axelsson & Lindhe (1981) | [38] | Surgery | PCS | 6 | 25 | MWF | PI, GI, PD, CAL | ||
| Crespi et al. (2011) | [35] | Surgery | RCT split | 15 | 25 | FOS+CO2L | MWF | PI, GI, PD, CAL | |
| Gaspirc & Skaleric (2007) | [57] | Surgery | RCT split | 5 | 25 | MWF+Er:YAG | MWF | GI, PI, BOP, PD, REC, CAL | |
| Isidor & Karring (1986) | [39] | Surgical/non-surgical | CCT | 5 | 16 | MWF | SRP | RBF | PI, GI, PD, CAL |
| Kaldahl et al. (1996) | [43] | Surgical/non-surgical | RCT | 7 | 51 | FOS | MWF | SRP, CSC | PI, PD, CAL, BOP, REC, SUP, TL |
| Kaldahl et al. (1996) | [31] | Surgical/non-surgical | RCT | 7 | 51 | FOS | MWF | SRP, CSC | PD, CAL |
| Lindhe et al. (1984) | [42] | Surgical/non-surgical | RCT split | 5 | 11 | MWF | SRP | CAL, PD | |
| Pihlstrom et al. (1983) | [32] | Surgical/non-surgical | RCT split | 6.5 | 10 | MWF | SRP | CAL, PD | |
| Pihlstrom et al. (1984) | [33] | Surgical/non-surgical | RCT split | 6.5 | 10 | MWF | SRP | CAL, PD, TL | |
| Preus et al. (2017) | [36] | Non-surgical | RCT | 5 | 161 | SRP+MET | SRP+PL | FMDIS+PL, FMDIS+MET | PI, BOP, PD, CAL |
| Ramberg et al. (2001) | [37] | Non-surgical | CCT | 5 | 115 | SRP+TTR | SRP | PI, BOP, CAL, PD, RX | |
| Ramfjord et al. (1975) | [56] | Surgical/non-surgical | RCT split | 5 | 79 | MWF | CUR | PE | CAL, PD |
| Knowles et al. (1979) | [30] | Surgical/non-surgical | RCT split | 8 | 43 | MWF | CUR | PE | CAL, PD |
| Ramfjord et al. (1987) | [41] | Surgical/non-surgical | RCT split | 5 | 72 | MWF | SRP | PE, CUR | CAL, PD, TL |
| Renvert et al. (1996) | [55] | Surgical/non-surgical | CS | 5 | 12 | MWF | SRP | CAL, PD, Micro | |
| Rosling et al. (2001) | [34] | Non-surgical | PCS | 12 | 334 | SRP | CAL, PD, PI, TL, RX | ||
| Serino et al. (2001) | [40] | Surgical/non-surgical | RCT | 13 | 64 | MWF | SRP | CAL, BOP, PD, RX |
SPT
Table 3
Periodontal disease progression as reported in the different studies

| Publication | SPT | PD | CAL | Frequency distributions |
|---|---|---|---|---|
| Axelsson & Lindhe (1981) [38] | One-third patients received maintenance by dentist (NRG). Two-third university program with 1 appointment per 2 mon the first 2 yr and then 1 appointment per 3 mon after (RG). | NRG: 1.8±0.24 (BS) to 2.9±0.51 (6 yr). | NRG: 3.7±1.11 (BS) to 5.5±1.11 (6 yr). | PD: 18% increase of 4–6 mm at 6 yr in NRG. |
| RG: 1.9±0.32 (BS) to 1.6±0.35 (6 yr). | RG: 4.2±0.90 (BS) to 4.0±0.93 (6 yr). | Among sites with CAL loss: NRG; 44% ≤1 mm, 55% 2–5 mm, and 1% ≥6 mm. RG; 99% ≤1 mm, 1% 2–5 mm. | ||
| Crespi et al. (2011) [35] | Patients were recalled every 2 wk for 3 mon and twice a yr thereafter. | Change BS-15 yr | Change BS-15 yr | NR |
| i) 1–4 mm; MWF: 0.61 mm, LT: 0.35 mm. | i) 1–4 mm; MWF: −0.39 mm, LT: −0.38 mm. | |||
| ii) 5–6 mm; MWF: 0.03 mm, LT: 0.3 mm. | ii) 5–6 mm; MWF: −0.24 mm, LT: 0.3 mm. | |||
| iii) ≥7 mm; MWF: 0.95 mm, LT: 0.4 mm. | iii) ≥7 mm; MWF: −0.94 mm, LT: 0.37 mm. | |||
| Gaspirc & Skaleric (2007) [57] | SPT 1/mon for 3 mon and at mon 6 post-surgery, then 1/6 mon during the remainder of the study. | MW: 2.92±0.7 mm (BS) to 2.91±0.55 mm (5 yr) | MW: 3.81±1.06 mm (BS) to 4.05±0.85 mm (5 yr) | NR |
| LT: 2.82±0.70 mm (BS) to 2.84±0.43 mm (5 yr) | LT: 3.66±0.81 mm (BS) to 3.97±0.89 mm (5 yr) | |||
| Isidor & Karring (1986) [39] | First yr 1 professional cleaning every 2 wk. | MWF: 2.3 mm (BS), 3.1 mm (5 yr). | Change BS-5 yr | Eighteen surfaces (7.1%) in MWF and 7 surfaces in SRP (2.9%) showed ≥2 mm loss of CAL. Overall 48 surfaces (4.9%) showed an attachment loss of ≥2 mm after 5 yr. |
| During 2nd yr 1/3 mon from then every 6 mon. | SRP: 3.1 mm (BS), 3.2 mm (5 yr). | MWF: −0.2mm, SRP: 0.4 mm | ||
| Kaldahl et al. (1996) [43] | 1/3 mon after surgery, plaque control, OHI, CSC, SRP if necessary. | Change BS-7 yr | Change BS-7 yr | Tooth loss: 19 originally treated with CSC, 21 with SRP, 20 with MWF, and 5 teeth treated with FOS were extracted due to probing depth progressing past the apex. |
| i) 1–4 mm; SRP: 0 mm, MWF: 0.2 mm, FOS: 0.5 mm. | i) 1–4 mm; SRP: −0.3 mm, MWF: −0.5 mm, FOS: 0.1 mm. | |||
| ii) 5–6 mm; SRP: 0.2 mm, MWF: 0.2 mm, FOS: 0.6 mm. | ii) 5–6 mm; SRP: 0 mm, MWF: −0.1 mm, FOS: 0.1 mm. | |||
| iii) ≥7 mm; SRP: −0.4 mm, MWF: 0.1 mm, FOS: 0.5 mm. | iii) ≥7 mm; SRP: 0.3 mm, MWF: 0.1 mm, FOS: 0.1 mm. | |||
| Kaldahl et al. (1996) [31] | One-third mon after surgery, plaque control, OHI, CSC, SRP if necessary. | Reported in Kaldahl et al. (1996) [43] | Reported in Kaldahl et al. (1996) [43] | Breakdown defined ≥3 mm CAL loss. 35 patients had ≤0.99% sites with breakdown, 34 patients 1%–3% sites, 8 patients 3%–6% sites. Patients having an incidence of a >3.00% were smokers at BS. |
| Lindhe et al. (1984) [42] | For first 6 months professional tooth cleaning once/2 wk, next 18 mon once/3 mon. After this SRP was avoided and SPT were every 4–6 mon. | MWF: ±1 mm, 76% sites; +2 mm, 14%; −2 mm, 7%. | MWF: ±1 mm, 84% sites; −2 mm, 9%; −2 mm, 5%. | CAL gains of ≥2 mm in 2% sites. CAL loss ≥2 mm in 10%–12% of sites. Patients with poor oral hygiene had 20% of sites loss ≥2 mm while in good oral hygiene only 2%–3% sites. |
| SRP: ±1 mm, 84% sites; −2 mm, 9%; +2 mm, 5%. | SRP: ±1 mm, 85% sites; −2 mm, 7%; +2 mm, 7%. | |||
| Pihlstrom et al. (1983) [32] | Three to 4 times per year. OHI, CSC and SRP for 1 hr. | MWF: 1–3 mm, 0.3mm; 4–6 mm, 0.4 mm; >7 mm, 0.7 mm. | MWF: 1–3 mm, 0.1 mm; 4–6 mm, 0.2 mm; >7 mm, −0.2 mm. | NR |
| SRP: 1–3 mm, 0.2 mm; 4–6 mm, 0.05 mm; >7 mm, 0.2 mm. | SRP: 1–3 mm, 0.1 mm; 4–6 mm, 0.3 mm; >7 mm, 0.3 mm. | |||
| Pihlstrom et al. (1984) [33] | Three to 4 times per yr. OHI, CSC and SRP for 1 hr. | Change BS-6.5 yr | Change BS-6.5 yr | NR |
| i) 4–6 mm: MWF: Mol: 0.7, non-Mol: −0.31. SRP: Mol: −0.21, non-Mol: 0.3. | 4–6 mm: MWF: Mol: −0.06, non-Mol: 0.41. SRP: Mol: 0.21, non-Mol: 0.29. | |||
| ii) >7 mm: MWF: Mol: 0.48, non-Mol: −0.3. SRP: Mol: 0.74, non-Mol: −0.45 | >7 mm: MWF: Mol: −0.38, non-Mol: −0.3. SRP: Mol: −0.13, non-Mol: 0.5 | |||
| Preus et al. (2017) [36] | SPT at 3, 6, and 12 mon after therapy, and every 6 mon after that. SPT consisted of CSC and SRP and sites that required it. | Change BS-5 yr | Change BS-5 yr | Trend for increase in the number of teeth with PPD ≥5 mm noted, occurrence of PPD ≥5 mm remained stable. |
| FMDIS-MET: 0.2 mm, FDIS: 0.15 mm, SRP+MET: 0.17 mm, SRP: 0.21 mm. | FMDIS-MET: −0.53 mm, FDIS: −0.47 mm, SRP+MET: −0.74 mm, SRP: −0.44 mm. | TL: None, 45%; 1 TL, 24%; 2 TL, 14%; 3 TL, 9%; ≥4 TL, 8%. | ||
| Ramberg et al. (2001) [37] | SPT 3–4 times per yr. | Change BS-13 yr | Change BS-13Y: TTR: −1mm, SRP: −1.1mm | PD; BS |
| TTR(test): 0.4 mm, SRP(control): 0.3mm | ≤3 mm: test, 43%; control, 49%; 4–6 mm: test, 42%; control, 39%; ≥7 mm: test, 15%; control, 12%. | |||
| 13 yr | ||||
| ≤3 mm: test, 57%; control, 61%; 4–6 mm: test, 35%; control, 32%; ≥7 mm: test, 8%; control, 7%. | ||||
| Ramfjord et al. (1975) [56] | SPT every 3 mon. | Change in interproximal mean PPD: CUR, −0.3mm; MWF, −0.4 mm; PEL, −0.58 mm. | Change in mean interproximal CAL: CUR, 0.38 mm; MWF, −0.02 mm; PEL, −0.06 mm. | NR |
| Knowles et al. (1979) [30] | SPT every 3 mon. | Overall change in PD: 1–3 mm, −0.35 mm; 4–6 mm, −0.3 mm; ≥7 mm, −0.2 mm. | Overall change in CAL: 1–3 mm, −0.3 mm; 4–6 mm, 0.25 mm; ≥7 mm, −0.4 mm. | NR |
| Ramfjord et al. (1987) [41] | Once per wk for 4 wk post-surgically, and later once 1/3 mon for 5 yr. | BS-5 yr | Change BS-5 yr in mm | % Sites CAL loss ≥2 mm |
| i) 1–3 mm; PEL: 0.43, CUR: 0.1, MWF: 0.17, SRP: 0.03. | i) 1–3 mm; PEL: −0.53, CUR: −0.64, MWF: −0.56, SRP: 0.62. | i) 1–3 mm, PEL: 38.1, CUR: 33.6, MWF: 35.4, SRP: 30.3. | ||
| ii) 4–6 mm; PEL: −0.52, CUR: −0.65, MWF: 0.39, SRP: 0.18. | ii) 4–6 mm; PEL: −0.49, CUR: −0.42, MWF: −0.43, SRP: −0.57. | ii) 4–6 mm; PEL: 29.3, CUR: 22.6, MWF: 27.9, SRP: 21.1. | ||
| iii) ≥7 mm; PEL: −0.64, CUR: 1.29, MWF: 0.28, SRP: −0.07. | iii) ≥7 mm; PEL: 0.26, CUR: 0.4, MWF: 0.5, SRP: 0.4. | iii) ≥7 mm; PEL: 10.8, CUR: 10.9, MWF: 8.1, SRP: 14.8. | ||
| Renvert et al. (1996) [55] | One-third mon for 3 yr and thereafter every 6 mon. Plaque scores, OHI, CSC, no SRP. | PD change BS-5 yr: 1.4 mm | CAL change BS-5 yr: −0.5 mm | NR |
| Rosling et al. (2001) [34] | Three to 4 times per year, according to individual needs. OHI, BOP, PD. | % of molar sites exhibiting an increase in PD ≥2 mm | CAL change: NG: 0.45 mm, HSG: 0.8 mm | NG 10% of subjects had 8 teeth exhibiting ≥2 mm CAL loss. 70% of HSG subjects had >8 teeth with ≥2 mm CAL change. |
| i) 0–3 mm; NG: 4.6%, HSG: 34.3%. | ||||
| ii) 4–5 mm; NG: 2.4%, HSG: 25.6%. | ||||
| iii) ≥6 mm; NG: 16.1%, HSG: 18.1%. | ||||
| Serino et al. (2001) [40] | SPT 4 times per year. Plaque control. Additional SRP at sites ≥5 mm. | PD change BS-13 yr | Mean annual CAL loss | Annual % of sites ≥2 mm CAL loss stratified by PD CAL loss in PD ≥6 mm |
| SRP: 0.6 mm, MWF: 0.6 mm. | i) SRP: 0.08 mm (1–3 yr), 0.11 mm (3–5 yr), and 0.07 mm (5–13 yr). | i) SRP: 7.5% (1–3 yr), 7.8% (3–5 yr), 2.9% (5–13 yr). | ||
| ii) MWF: 0.11 mm (1–3 yr), 0.07mm (3–5 yr), 0.07mm (5–13 yr). | ii) MWF: 5% (1–3 yr), 4% (3–5 yr), 2.3% (5–13 yr). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download