Abbreviations
BMDM
cDNA
DMEM
EKSA
HDAC
iNOS
LB
LTA
MPN
MTT
NO
PAMP
P-STAT1
PTX
Sa.LPP
SAHA
SCFA
TBS
TBST
TSA
INTRODUCTION
MATERIALS AND METHODS
Bacteria, reagents, and chemicals
Preparation of ethanol-killed S. aureus (EKSA)
Culture of RAW 264.7 cells
Preparation of bone marrow-derived macrophages (BMDMs)
Measurement of NO production
Cell viability assay
Western blotting
RT-PCR
Transient transfection and reporter gene assay
Preparation of lipoproteins from S. aureus
Statistical analysis
RESULTS
Butyrate and propionate, but not acetate, inhibit Sa.LPP-induced NO production in RAW 264.7 cells
Figure 1
Butyrate and propionate, but not acetate, inhibit S. aureus or its lipoprotein-induced NO production in murine macrophages. (A) RAW 264.7 cells (3×105 cells/ml) were treated with 1×108 CFU/ml of live S. aureus in the presence (0.3, 1, or 3 mM) or absence of acetate, propionate, or butyrate for 3 h and further incubated with 200 μg/ml of gentamicin for 21 h. (B) RAW 264.7 cells (3×105 cells/ml) were treated with 50 μg/ml of EKSA in the presence (0.3, 1, or 3 mM) or absence of acetate, propionate, or butyrate for 24 h. (C) RAW 264.7 cells (3×105 cells/ml) were stimulated with 5 μg/ml of Sa.LPP in the presence (0.3, 1, or 3 mM) or absence of acetate, propionate, or butyrate for 24 h. Nitrite in the culture supernatant was measured to determine NO concentration using the Griess reagent. (D) BMDMs (5×105 cells/ml) were stimulated with 5 μg/ml of Sa.LPP in the presence or absence of 3 mM acetate, propionate, or butyrate for 24 h, and nitrite was measured. (E) RAW 264.7 cells (3×105 cells/ml) were treated with 5 μg/ml of Sa.LPP and cultured in the presence (0.3, 1, or 3 mM) or absence of acetate, propionate, or butyrate for 24 h. Cell viability was measured by MTT assay. Data are mean values±standard deviations of 3 independent experiments.

Butyrate and propionate, but not acetate, inhibit Sa.LPP-induced iNOS expression in RAW 264.7 cells
Figure 2
Butyrate and propionate, but not acetate, inhibit Sa.LPP-induced iNOS expression in RAW 264.7 cells. (A) RAW 264.7 cells (3×105 cells/ml) were treated with 5 μg/ml of Sa.LPP in the presence or absence of 3 mM acetate, propionate, or butyrate for 15 h. Cell lysates were analyzed to measure iNOS or β-actin expression by Western blotting. (B) RAW 264.7 cells (3×105 cells/ml) were treated with 5 μg/ml of Sa.LPP in the presence or absence of 3 mM acetate, propionate, or butyrate for 12 h. Total RNA was isolated, and the mRNA expression levels of iNOS or β-actin was examined by RT-PCR. Relative expression levels of iNOS protein and mRNA to those of β-actin were measured to obtain mean values±standard deviations by densitometry from three independent experiments (right panels).
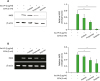
Butyrate and propionate inhibit Sa.LPP-induced NF-κB activation, STAT1 phosphorylation, and IFN-β mRNA expression in RAW 264.7 cells
Figure 3
Butyrate and propionate, but not acetate, inhibit Sa.LPP-induced NF-κB activation, STAT1 phosphorylation, and IFN-β mRNA expression in RAW 264.7 cells. (A) RAW 264.7 cells (1×106 cells/ml) were transfected with a firefly luciferase reporter plasmid regulated by NF-κB for 3 h. After the cells were harvested and plated on 96-well plates for 3 h, the cells were treated with 5 μg/ml of Sa.LPP and cultured in the presence or absence of 3 mM acetate, propionate, or butyrate for additional 21 h. Cell lysates were mixed with firefly luciferase substrate to determine luciferase activity. (B) RAW 264.7 cells (3×105 cells/ml) were treated with 5 μg/ml of Sa.LPP in the presence or absence of 3 mM acetate, propionate, or butyrate for 3 h. Cell lysates were analyzed to measure phosphorylated NF-κB p65 or β-actin by Western blotting. (C) RAW 264.7 cells (3×105 cells/ml) were treated with 5 μg/ml of Sa.LPP in the presence or absence of 3 mM acetate, propionate, or butyrate for 3 h. Cell lysates were analyzed to measure phosphorylated STAT1, nonphosphorylated STAT1 or β-actin by Western blotting. (D) RAW 264.7 cells (5×105 cells/ml) were treated with 5 μg/ml of Sa.LPP in the presence or absence of 3 mM acetate, propionate, or butyrate for 6 h. Total RNA was isolated, and the mRNA expression level of IFN-β was examined by RT-PCR. Relative expression levels of P-STAT1 to STAT1 and IFN-β to β-actin were measured to obtain mean values±standard deviations by densitometry from 3 independent experiments (right panels).
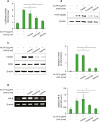
Butyrate inhibits Sa.LPP-induced NO production by inhibiting HDAC
Figure 4
Butyrate and propionate might inhibit Sa.LPP-induced NO production by inhibiting HDAC. (A) RAW 264.7 cells (3×105 cells/ml) were pre-treated with HDAC inhibitors (0.5 μM SAHA or 0.05 μM TSA) or SCFAs (3 mM of acetate, propionate, or butyrate) for 1 h and subsequently stimulated with 5 μg/ml of Sa.LPP for additional 23 h. Nitrite in the culture supernatant was measured to determine NO concentration using the Griess reagent. (B) RAW 264.7 cells (3×105 cells/ml) were pre-treated with 0.01 or 0.05 μM of TSA for 1 h and subsequently stimulated with 5 μg/ml of Sa.LPP for additional 3 h. Cell lysates were analyzed to measure phosphorylated STAT1, nonphosphorylated STAT1, phosphorylated NF-κB p65 or β-actin by Western blotting. (C) RAW 264.7 cells (5×105 cells/ml) were pre-treated with 0.01 or 0.05 μM of TSA for 1 h and subsequently stimulated with 5 μg/ml of Sa.LPP for an additional 6 h. Total RNA was isolated, and the mRNA expression level of IFN-β was examined by RT-PCR. (D) RAW 264.7 cells (3×105 cells/ml) were treated with 5 μg/ml of Sa.LPP in the presence or absence of acetate, propionate, or butyrate for 4 h. Cell lysates were obtained and analyzed by Western blotting to measure histone acetylation at lysine residues. (E, F) RAW 264.7 cells (3×105 cells/ml) were pre-treated with (E) 0.01 μM of MPN, or (F) 0.01 μg/ml of PTX for 1 h and subsequently stimulated with 5 μg/ml of Sa.LPP for an additional 23 h. Nitrite in the culture supernatant was measured to determine NO concentration using the Griess reagent. Data are mean values±standard deviation of 3 independent experiments.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download