INTRODUCTION
MATERIALS AND METHODS
Mice
Bone marrow, spleen, lymph node, and blood isolation
Flow cytometry
BrdU incorporation assay
Bone marrow reconstitution
RESULTS
Atg5 deficiency in hematopoietic cells causes survival defects in mice
 | Figure 1Vav_Atg5−/− mice display defects in survival, body weights, and total hematopoietic cell number. Starting 3 wk after birth, survival (A) and body weights (B) of newborn control and Vav_Atg5−/− pups were monitored every wk. (C) The number of bone marrow, spleen, and lymph node cells from these animals were counted (n=3). Data are representative of 3 independent experiments and presented as mean ± SEM.
*p<0.05, **p<0.01, ***p<0.001 as calculated by Student's t-test.
|
Hematopoietic cell-specific Atg5-deficient mice suffer from severe lymphopenia
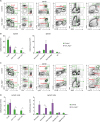 | Figure 2Vav_Atg5−/− mice suffer from severe lymphopenia. Spleen (A, B) and lymph nodes (C, D) were isolated from control and Vav_Atg5−/− mice. B cells (CD3−B220+), CD4 (CD3+CD4+) T cells, CD8 (CD3+CD8a+) T cells, dendritic cells (DC; B220−CD11C+MHC class II+) in spleen, resident DCs (B220−CD11c+MHC class IIInt) and migratory DCs (B220−CD11c+MHC class IIHigh) in lymph nodes, neutrophils (CD11b+Ly6G+), monocytes (CD11b+Ly6CHigh), and macrophage (CD11b+F4/80+) were classified by flow cytometry. Representative FACS plots (A, C) and absolute cell numbers (B, D) were calculated (n=3). Data are representative of three independent experiments and presented as mean ± SEM.
*p<0.05 as calculated by Student's t-test.
|
Atg5 deficiency results in defective erythropoiesis
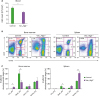 | Figure 3Erythropoiesis is altered following Atg5 deficiency. (A) The number of RBCs in peripheral blood were counted in control and Vav_Atg5−/− mice. (B) Erythroid developmental stages in bone marrow and spleen were assessed by flow cytometry. Pro-erythroblasts (Pro_ery; Ter119−CD71High), basophilic erythroblasts (Baso_ery; Ter119+CD71High), chromatophilic erythroblasts (Chro_ery; Ter119+CD71Med), and orthochromatophilic erythroblasts (Ortho_ery; Ter119+CD71−) were characterized (n=4). Data are representative of three independent experiments and presented as mean ± SEM.
*p<0.05 and **p<0.01 as calculated by Student's t-test.
|
Atg5 is essential for the HSCs maintenance and reconstitution ability
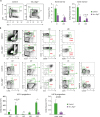 | Figure 4Atg5 deficiency leads to defective maintenance of HSCs and developmental impairment of hematopoietic progenitor cells. (A, B) Bone marrow cells isolated from 11-wk-old from control and Vav_Atg5−/− mice were analyzed. (A) Frequency and absolute number of LK and LSK cells were assessed by flow cytometry (n=4–5). (B) HSCs were characterized as LSK CD48−CD150+, and multiple progenitor cells were characterized in CLPs (Lin−IL-7Ra+Flt3+), CCR9+ LMPPs (LSK Flt3+), NKPs (Lin−CD122+NK1.1−DX5−), MkPs (LK CD41+CD150+), and GMPs (LK CD41−FcgRII/III+) (n=3). Data are representative of three independent experiments and presented as mean ± SEM.
*p<0.05, **p<0.01, ***p<0.001 as calculated by Student's t-test.
|
 | Figure 5Hematopoietic cell specific Atg5 deficiency results in aberrant proliferation and mitochondrial dysfunction in hematopoietic cells. (A) Apoptosis and cell proliferation assays of LSKs from control and Vav_Atg5−/− mice were performed using Annexin V, BrdU, and Ki67 staining. For the BrdU assay, proliferation of LSK cells was analyzed 24 h after BrdU injection (n=4–5). (B) Approximately 2×106 of total bone marrow cells from control and Vav_Atg5−/− mice were injected intravenously into lethally irradiated CD45.1+ mice. After 8 weeks, percentage of reconstitution of transplanted bone marrow in peripheral blood was confirmed by flow cytometry (n=3). (C) Mitochondrial functions in bone marrow LSK cells from control and Vav_Atg5−/− mice were assessed using MitoTracker Green, MitoTracker Red, and MitoSox (n=4–5). Data are representative of 3 independent experiments and presented as mean ± SEM.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 as calculated by Student's t-test.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download