INTRODUCTION
MATERIALS AND METHODS
Mice and viral infection
Administration of IM156 and rapamycin to mice
Cell isolation, antibodies, and flow cytometry
RESULTS
IM156 inhibits the memory differentiation of antigen-specific CD8+ T cells
 | Figure 1IM156 reduces antigen-specific effector memory T cells in LCMV-infected mice.LCMV-infected mice were treated with DMSO (
 ), IM156 ( ), IM156 ( ), or rapamycin ( ), or rapamycin ( ) from days −1 to 29 post-infection. (A) Representative plot showing the frequency of GP33 tetramer-positive cells among CD8+ or CD4+ T cells in PBMCs at the indicated days post-infection. (B) Kinetics of GP33+ T cells among CD8+ T cells as in A. (C) Co-expression of either KLRG1 (left) or CD62L (right) and CD127 on tetramer-positive CD8+ T cells obtained from PBMCs at the indicated days post-infection. (D) Frequency of MPECs (CD127hiCD62Llo) and SLECs (CD127loCD62Lhi) as in C. (E) Frequency of TCM (CD127+CD62L+), TEM (CD127+CD62L−), and TEFF (CD127−CD62L−) as in C. Data are representative of 3 independent experiments with n=4 mice per group. Results are the mean ± SEM and statistical significance was determined by 2-tailed unpaired Student's t-test. ) from days −1 to 29 post-infection. (A) Representative plot showing the frequency of GP33 tetramer-positive cells among CD8+ or CD4+ T cells in PBMCs at the indicated days post-infection. (B) Kinetics of GP33+ T cells among CD8+ T cells as in A. (C) Co-expression of either KLRG1 (left) or CD62L (right) and CD127 on tetramer-positive CD8+ T cells obtained from PBMCs at the indicated days post-infection. (D) Frequency of MPECs (CD127hiCD62Llo) and SLECs (CD127loCD62Lhi) as in C. (E) Frequency of TCM (CD127+CD62L+), TEM (CD127+CD62L−), and TEFF (CD127−CD62L−) as in C. Data are representative of 3 independent experiments with n=4 mice per group. Results are the mean ± SEM and statistical significance was determined by 2-tailed unpaired Student's t-test.
*p<0.05; **p<0.01; ***p<0.001.
|
IM156 delays conversion of CD127 and CD62L of antigen-specific CD8+ T cell in a dose-dependent manner
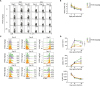 | Figure 2IM156 dose-dependently impairs CD127 conversion of antigen-specific CD8+ T cells.LCMV-infected mice were treated with various concentrations of IM156 from days −1 to 29 post-infection. (A) Representative plot showing the frequency of GP33 tetramer-positive cells among CD8+ or CD4+ T cells in PBMCs of IM156-treated mice at the indicated days post-infection. (B) Frequency of GP33+CD8+ T cells as in A. (C) Expression levels of CD127 (left), CD62L (middle), and KLRG1 (right) on tetramer-positive CD8+ T cells in PBMCs of IM156-treated mice at the indicated time post-infection. The relative percentages of CD127, CD62L, and KRLG1-expressing cells are indicated. (D) MFI of CD127 (top), CD62L (middle), and KLRG1 (bottom) on virus-specific CD8+ T cells in PBMCs as in C.
Data are representative of 3 independent experiments n=4–5 mice per group. Results are the mean ± SEM and statistical significance was determined by two-tailed unpaired Student's t-test.
*p<0.05; **p<0.01; ***p<0.001.
|
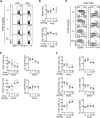 | Figure 3IM156 dose-dependently suppresses the differentiation of central memory T cells and effector memory T cells.LCMV-infected mice were treated with various concentrations of IM156 from days −1 to 29 post-infection. (A) Representative data showing the frequency of GP33 tetramer-positive cells among CD8+ or CD4+ T cells in the spleen at day 30 post-infection. (B) Frequency (top) and absolute number (bottom) of GP33+CD8+ T cells as in A. (C) Co-expression of either KLRG1 (left) or CD62L (right) and CD127 on tetramer-positive CD8+ T cells obtained from the spleen at day 30 post-infection. (D) Frequency (left) and absolute number (right) of MPECs (CD127hi KLRG1lo) and SLECs (CD127loKLRG1hi) as in C. (E) Frequency (left) and absolute number (right) of TCM (CD127+CD62L+), TEM (CD127+CD62L−), and TEFF (CD127−CD62L−) as in C. Data are representative of 3 independent experiments n=4–5 mice per group. Results are the mean ± SEM and statistical significance was determined by 2-tailed unpaired Student's t-test.
*p<0.05; **p<0.01; ***p<0.001.
|
IM156 dose-dependently increases SLECs but decreases MPECs
IM156 impairs the ability of antigen-specific CD8+ T cells to produce cytokines
 | Figure 4IM156 inhibits multi-functional CD8+ and CD4+ T cells.LCMV-infected mice were treated with various concentrations of IM156 from days −1 to 29 post-infection. (A) Representative flow cytometric analysis of production of IFN-γ, TNF-α, and IL-2 in CD8+ T cells obtained from the spleen at day 30 post-infection after ex vivo re-stimulation with GP33 peptide. (B) Frequency of TNF-α-producing cells (top left) and IL-2-producing cells (top right) among IFN-γ+CD8+ T cells. MFI of TNF-α (bottom left) and IL-2 (bottom right) among IFN-γ+CD8+ T cells as in A. (C) Representative flow cytometric analysis of production of IFN-γ, TNF-α, and IL-2 in CD4+ T cells obtained from the spleen at day 30 post-infection after ex vivo re-stimulation with GP66 peptide. (D) Frequency of TNF-α-producing cells (top left) and IL-2-producing cells (top right) among IFN-γ+CD4+ T cells. MFI of TNF-α (bottom left) and IL-2 (bottom right) among IFN-γ+CD4+ T cells as in C. Data are representative of 3 independent experiments n=4–5 mice per group. Results are the mean ± SEM and statistical significance was determined by 2-tailed unpaired Student's t-test.
*p<0.05; **p<0.01; ***p<0.001.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download