Abstract
Purpose
This review aimed to determine the effectiveness of oral care using Chlorhexidine Gluconate(CHX) in Ventilator-Associated Pneumonia (VAP) in the intensive care unit.
Methods
An electronic databases search was conducted with Ovid-MEDLINE, EMBASE, CENTRAL, CINAHL and four domestic databases from July 10 to 16, 2018. Two reviewers independently selected the studies; three reviewers assessed their methodological quality and extracted relevant data. We conducted a metaanalysis of the effect of CHX oral care versus placebo using the Review Manager 5.3 software program and summarized the results of intervention from the included studies.
Results
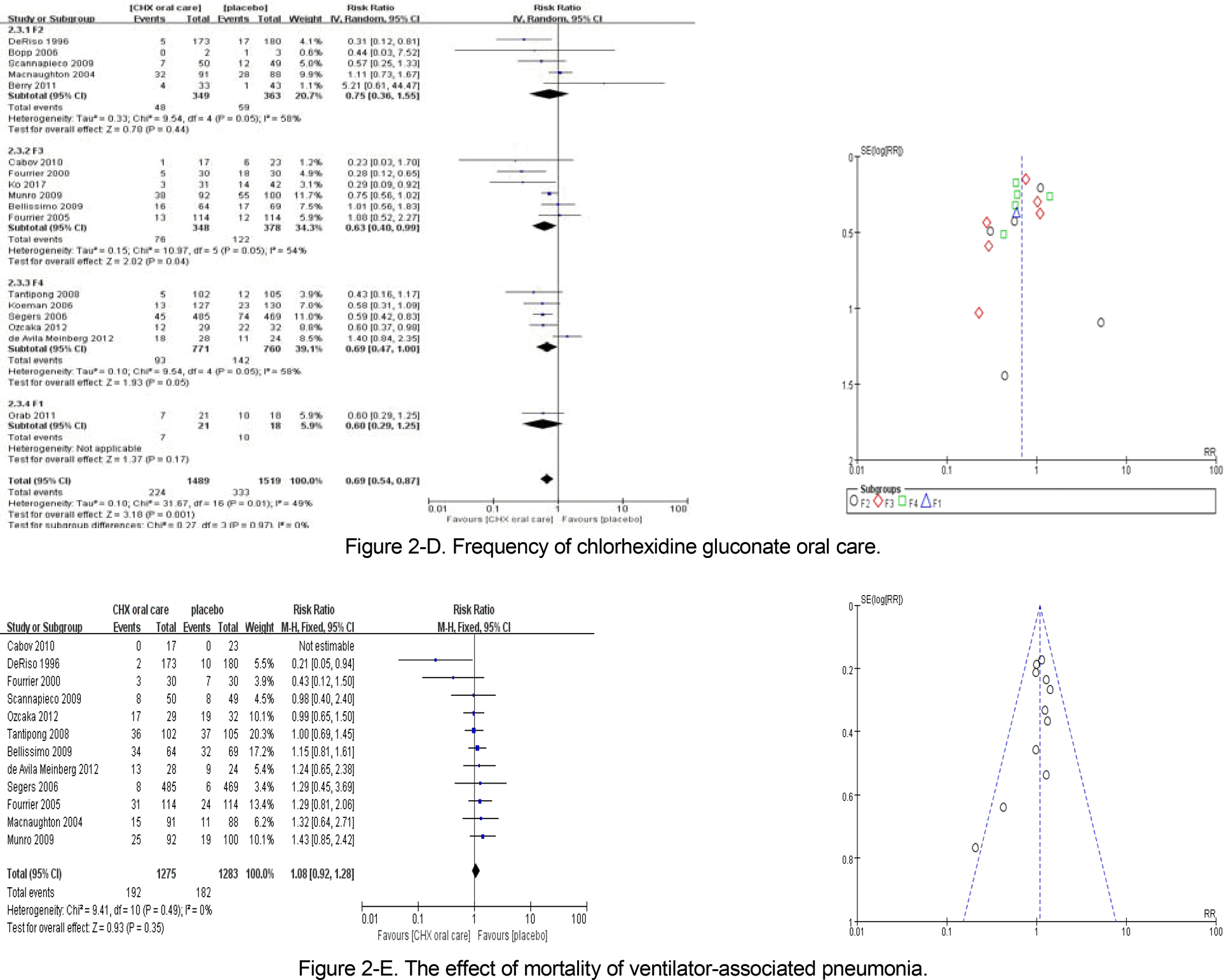
Of the 512 articles identified, 17 randomized controlled trials met the inclusion criteria for review. The incidence of VAP differed significantly between the CHX and placebo groups(Relative Risk [RR]=0.72, 95% Confidence Interval [CI]=0.63~0.84). The pooled effects of oral care using 0.12% CHX were RR=0.65(95% CI=0.52~0.80) and RR=0.68 (95% CI=0.54~0.86) using CHX solution, which were statistically significant. When CHX oral care was performed three times a day, the size of the effect was statistically significant (RR=0.63, 95% CI=0.40~0.99). There was no significant difference in mortality between the CHX oral care and placebo groups (RR=1.08, 95% CI=0.94~1.28).
Conclusion
This review provides evidence that performing oral care using a 0.12% CHX solution three times a day could decrease the incidence of VAP. For improving the quality of nursing practice, the results of this review should be used as the basis for the oral care evidence-based practice guidelines for critical patients.
Go to : 
REFERENCES
1. Bouadma L, Klompas M. Oral care with chlorhexidine: be-ware! Intensive Care Medicine. 2018; 44(7):1153–5. https://doi.org/10.1007/s00134-018-5221-x.

2. Kwak YG, Choi YH, Choi JY, Yoo HM, Lee S-O, Kim HB, et al. Korean national healthcare-associated infections surveillance system, intensive care unit module report: summary of data from July 2015 through June 2016. Korean Journal of Healthcare-Associated Infection Control and Prevention. 2017; 22(1):920. https://doi.org/10.14192/kjhaicp.2017.22.1.9.

3. Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator- associated pneumonia. Cochrane Database of Systematic Review. 2016; 10:1–136. https://doi.org/10.1002/14651858.CD008367.pub3.
4. Kim NY, Kim YH, Kim EA, Ru SA, Park SJ, Yang JJ, et al. AACN essentials of critical care nursing. 3rd ed. Seoul: Hyun-moonsa;2016. p. 182.
5. Munro CL, Grap MJ, Elswick RK, McKinney J, Sessler CN, Hummel RS. Oral health status and development of ventilator- associated pneumonia: a descriptive study. American Journal of Critical Care. 2006; 15(5):453–60.
6. Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing healthcare-associated pneumonia: recommendations of CDC and the healthcare infection control practices advisory committee. Morbidity and Mortality Weekly Re-port. 2004; 53(RR-3):8–9. https://doi.org/10.1037/e548652006-001.
7. Americam Association of Critical-Care Nurses. Prevention of ventilator-associated pneumonia in adults. Critical Care Nurse. 2017; 37(3):e22–5. https://doi.org/10.4037/ccn2017460.
8. Alhazzani W, Smith O, Muscedere J, Medd J, Cook D. Tooth-brushing for critically ill mechanically ventilated patients: a systematic review and metaanalysis of randomized trials evaluating ventilator-associated pneumonia. Critical Care Medicine. 2013; 41(2):646–55. https://doi.org/10.1097/ccm.0b013e3182742d45.
9. Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and metaanalysis. JAMA Internal Medicine. 2014; 174(5):751–61. https://doi.org/10.1001/jamainternmed.2014.359.
10. Chan EY, Ruest A, Meade MO, Cook DJ. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and metaanalysis. BMJ. 2007; 334(7599):889. https://doi.org/10.1136/bmj.39136.528160.be.

11. Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and metaanalysis. The Lancet Infectious Diseases. 2011; 11(11):845–54. https://doi.org/10.1016/s1473-3099(11)70127-x. https://doi.org/10.1016/s1473-3099(11)70127-x.

12. Deschepper M, Waegeman W, Eeckloo K, Vogelaers D, Blot S. Effects of chlorhexidine gluconate oral care on hospital mortality: a hospital-wide, observational cohort study. Intensive Care Medicine. 2018; 44(7):1017–26. https://doi.org/10.1007/s00134-018-5171-3.

13. Lee JY. Meta-analysis 1, 2, 3. Paper presented at: The meeting of the Korean Society of Evidence-Based Nursing. 2019. Janu-ary 22; Seoul, Korea.
14. Feider LL, Mitchell P, Bridges E. Oral care practices for orally intubated critically ill adults. American Journal of Critical Care. 2010; 19(2):175–83. https://doi.org/10.4037/ajcc2010816.

15. Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. Journal of Critical Care. 2008; 23(1):126–37. https://doi.org/10.1016/j.jcrc.2007.11.014.

16. Choi KB, Mo HS, Kim JS. Survey of oral health care practices for intubated patients by intensive care unit nurses. Health & Nursing Sciences. 2009; 21(1):1–12.
17. Bidwell S, Jensen MF. Using a search protocol to identify sources of information. Topfer LA, Auston I, editors. Etext on health technology assessment information resources [Internet]. Rock-ville Pike (MD): National Library of Medicine;2006. [cited 2018 Sep 10]. Available from:. https://www.nlm.nih.gov/archive/20060905/nichsr/ehta/chapter3.html#COSI. https://www.nlm.nih.gov/archive/20060905/nichsr/ehta/chapter3.html#COSI.
18. Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool to assess risk of bias in randomized trials (RoB 2.0) [Internet]. Bristol: Authors;2016. [cited 2018 Sep 16]. Available from:. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool.
19. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Internet]. London (UK): The Cochrane Collaboration;2011. [cited 2018 Sep 16]. Available from:. http://handbook.cochrane.org.
20. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002. https://doi.org/10.1136/bmj.d4002.

21. Song UR, Kim SY. Effects of ventilator-associated pneumonia prevention program on incidence rate and endotracheal colonization. Korean Journal of Adult Nursing. 2017; 28(6):628–36. https://doi.org/10.7475/kjan.2016.28.6.628.
22. Zhang T-T, Tang S-S, Fu L-J. The effectiveness of different concentrations of chlorhexidine for prevention of ventilator-associated pneumonia: a metaanalysis. Journal of Clinical Nursing. 2014; 23(11-12):1461–75. https://doi.org/10.1111/jocn.12312.

23. Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a metaanalysis. Critical Care Medicine. 2007; 35(2):595–602. https://doi.org/10.1097/01.CCM.0000253395.70708.AC.

24. Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults' intensive care units. Journal of Critical Care. 2017; 40:318–22. https://doi.org/10.1016/j.jcrc.2017.02.029.

25. Grap MJ, Munro CL. Preventing ventilator-associated pneumonia: evidence-based care. Critical Care Nursing Clinics of North America. 2004; 16(3):349–58. https://doi.org/10.1016/j.ccell.2004.03.005.

26. Berry AM, Davidson PM. Beyond comfort: oral hygiene as a critical nursing activity in the intensive care unit. Intensive and Critical Care Nursing. 2006; 22(6):318–28. https://doi.org/10.1016/j.iccn.2006.04.003.

27. An JH. A study on the awareness of ICU nurses about mouth care and the state of their mouth care [master's thesis]. Busan: Dong-A University;2006. p. 20.
28. Hutchins K, Karras G, Erwin J, Sullivan KL. Ventilator-associated pneumonia and oral care: a successful quality improvement project. American Journal of Infection Control. 2009; 37(7):590–7. https://doi.org/10.1016/j.ajic.2008.12.007.

29. Ross A, Crumpler J. The impact of an evidence-based practice education program on the role of oral care in the prevention of ventilator-associated pneumonia. Intensive and Critical Care Nursing. 2007; 23(3):132–6. https://doi.org/10.1016/j.iccn.2006.11.006.

30. Siempos II, Vardakas KZ, Kyriakopoulos CE, Ntaidou TK, Falagas ME. Predictors of mortality in adult patients with ven-tilator-associated pneumonia: a metaanalysis. Shock. 2010; 33(6):590–601. https://doi.org/10.1097/SHK.0b013e3181cc0418.
Appendix 1. Studies included in a Systematic Review
A1. Bellissimo-Rodrigues F, Bellissimo-Rodrigues WT, Viana JM, Teixeira GCA, Nicolini E, Auxiliadora-Martins M, et al. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infection Control & Hospital Epidemiology. 2009; 30(10):952–8. https://doi.org/10.1086/605722.

A2. Berry AM, Davidson PM, Masters J, Rolls K, Ollerton R. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: a randomised control trial. International Journal of Nursing Studies. 2011; 48(6):681–8. https://doi.org/10.1016/j.ijnurstu.2010.11.004.

A3. Bopp M, Darby M, Loftin KC, Broscious S. Effects of daily oral care with 0.12% chlorhexidine gluconate and a standard oral care protocol on the development of nosocomial pneumonia in intubated patients: a pilot study. Journal of Dental Hygiene. 2006; 80(3):1–13.
A4. Ć abov T, Macan D, Husedžinović , Škrlin-Šubić J, Bošnjak D, Šestan-Crnek S, et al. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: a randomized placebo-controlled study. Wiener Klinische Wochenschrift. 2010; 122(13-14):397–404. https://doi.org/10.1007/s00508-010-1397-y. A5. DeRiso II AJ, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and non-prophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996; 109(6):1556–61. https://doi.org/10.1378/chest.109.6.1556.
A6. Fourrier F, Cau-Pottier E, Boutigny H, Roussel-Delvallez M, Jourdain M, Chopin C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Medicine. 2000; 26(9):1239–47.

A7. Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmet-tre T, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the inten sive care unit: a double-blind placebo-controlled multicenter study. Critical Care Medicine. 2005; 33(8):1728–35. https://doi.org/10.1097/01.CCM.0000171537.03493.B0.
A8. Grap MJ, Munro CL, Hamilton VA, Elswick Jr. RK, Sessler CN, Ward KR. Early, single chlorhexidine application reduces ventilator-associated pneumonia in trauma patients. Heart & Lung. 2011; 40(5):e115–22. https://doi.org/10.1016/j.hrtlng.2011.01.006.
A9. Ko M, Kim J, Choi E. The effect of oral care with 0.12% chlorhexidine for VAP and oral health in critically ill patients. Asia Pacific Journal of Multimedia Services Convergent with Art, Humanities, and Sociology. 2017; 7(3):461–76. https://doi.org/10.14257/AJMAHS.2017.03.08.

A10. Koeman M, van der Ven AJAM, Hak E, Joore HCA, Kaasja-ger K, de Smet AGA, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. American Journal of Respiratory and Critical Care Medicine. 2006; 173(12):1348–55. https://doi.org/10.1164/rccm.200505-820OC.

A11. Macnaughton PD, Bailey J, Donlin N, Branfield P, Williams A, Rowswell H. A randomised controlled trial assessing the efficacy of oral chlorhexidine in ventilated patients. Intensive Care Medicine. 2004; 30(suppl 1):S12.
A12. Meinberg MCDA, Cheade MDFM, Miranda ALD, Fachini MM, Lobo SM. The use of 2% chlorhexidine gel and tooth-brushing for oral hygiene of patients receiving mechanical ventilation: effects on ventilator-associated pneumonia. Re-vista Brasileira de Terapia Intensiva. 2012; 24(4):369–74. https://doi.org/10.1590/S0103-507X2012000400013.
A13. Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associ-ated pneumonia in critically ill adults. American Journal of Critical Care. 2009; 18(5):428–37. https://doi.org/10.4037/ajcc2009792.

A14. Özçaka Ö, Baş oğ lu Ö K, Buduneli N, Taş bakan MS, Bacakoğ lu F, Kinane DF. Chlorhexidine decreases the risk of ventilator- associated pneumonia in intensive care unit patients: a randomized clinical trial. Journal of Periodontal Research. 2012; 47(5):584–92. https://doi.org/10.1111/j.1600-0765.2012.01470.x.
A15. Scannapieco FA, Yu J, Raghavendran K, Vacanti A, Owens SI, Wood K, et al. A randomized trial of chlorhexidine gluconate on oral bacterial pathogens in mechanically ventilated patients. Critical Care. 2009; 13(4):R117. https://doi.org/10.1186/cc7967.

A16. Segers P, Speekenbrink RGH, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and orophar-ynx with chlorhexidine gluconate: a randomized controlled trial. The Journal of the American Medical Association. 2006; 296(20):2460–6. https://doi.org/10.1001/jama.296.20.2460.
A17. Tantipong H, Morkchareonpong C, Jaiyindee S, Thamlikitkul V. Randomized controlled trial and metaanalysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infection Control & Hospital Epidemiology. 2008; 29(2):131–6. https://doi.org/10.1086/526438.

Go to : 
Table 1.
Descriptive Summary of Included Studies and Risk of Bias Summary
C=control; CDC=centers for disease control and prevention; CHX=chlorhexidine; CPIS=clinical pulmonary infection score; I=intervention; R=randomization process; D=deviations from intended interventions; M1=missing outcome data; M2=measurement of the outcome; mini-BAL=mini-bronchoalveolar lavage; NR= not reported; O=overall Bias; S=selection of the reported result; VAP=ventilator-associated pneumonia; Ⓛ=low risk of bias; Ⓗ=high risk of bias; Ⓢ=some concern.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download