INTRODUCTION
METHODS
Patients
Anesthetic monitoring and maintenance
Increments in the Ce of propofol and TEE image recording
Off-line analyses of recorded TEE images
Pharmacodynamic modelling for predicting the Ce of propofol producing an Sm decline
Statistical analyses
Ethics statement
RESULTS
Table 1
Demographic and preoperative echocardiographic indexes (n = 33)

Table 2
Serial hemodynamic and transesophageal echocardiographic data during the increments in Ce of propofol

TEE data
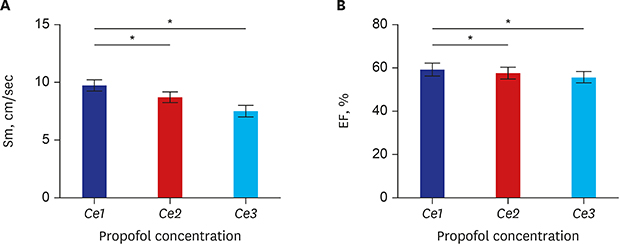
 | Fig. 1Serial changes in (A) Sm and (B) EF with increments in Ce of propofol from Ce1 to Ce3. (A) As propofol concentration increases from Ce1 (0.8 ± 0.1) to Ce2 (1.6 ± 0.2) and Ce3 (2.4 ± 0.3), Sm was decreased. (B) As propofol concentration increases from Ce1 (0.8 ± 0.1) to Ce2 (1.6 ± 0.2) and Ce3 (2.4 ± 0.3), EF was decreased. The error bars indicate the standard error at each concentration.Sm = peak systolic velocity of the mitral annulus descending toward the apex, EF = ejection fraction, Ce = effect-site concentration.
*P < 0.05.
|
 | Fig. 2Serial changes in (A) EDV, (B) ESV, (C) Ea, (D) SVRI with increments in Ce of propofol from Ce1 to Ce3. While (A) EDV and (D) SVRI was decreased as effect site of concentration of propofol increases, (B) ESV was significantly increased. (C) Ea was maintained during the entire range of propofol's effect site concentrations. The changes in EDV, EA, and SVRI according to concentration were not significant. The error bars indicates the standard error.EDV = end diastolic volume, ESV = end systolic volume, Ea = total arterial elastance, SVRI = systemic vascular resistance index, Ce = effect-site concentration.
*P < 0.05.
|
Table 3
Regression equation of indicators of left ventricular systolic performance and atrial contractile function according to the changes in the effect-site concentration of propofol

| Variables | Intercept (SE) | P value | Slope (SE) | P value |
|---|---|---|---|---|
| Sm | 10.83 (0.75) | < 0.01 | −1.01 (0.26) | < 0.01 |
| a′ | 0.02 (0.01) | 0.03 | 0.04 (0.01) | < 0.01 |
| LVEF | 61.34 (1.46) | < 0.01 | −2.24 (0.57) | < 0.01 |
Population pharmacodynamic parameter estimates for the Ce producing a reduction in BIS and Sm values
 | Fig. 3The relationship between the probability of a 10% (A) and 20% (B) decrease in the Sm from baseline and the Ce of propofol. (A) The estimate of the Ce50 (Ce50−10%, the effect-site concentration of propofol associated with a 50% probability of a decrease in the Sm) with RSE and interindividual variability presented as % coefficient variation was 1.4 μg/mL (13.3, 39.9%). The mean Ce95 (Ce95−10%, the effect-site concentration of propofol associated with a 95% probability of a decrease in the Sm) was 3.86 μg/mL for a 10% decrease in the Sm. The estimate of γ (the steepness of the concentration-vs.-response relation) with RSE and interindividual variability presented as % coefficient variation was 2.9 (43.1, 40.1%) for a 10% decrease in the Sm. Interindividual random variability was modeled using a log-normal model. (B) The estimate of the Ce50 (Ce50−20%, the effect-site concentration of propofol associated with a 50% probability of a decrease in the Sm) with RSE and interindividual variability presented as % coefficient variation was 2.14 μg/mL (12.5, 42.7%). The mean Ce95 (Ce95−20%, the effect-site concentration of propofol associated with a 95% probability of a decrease in the Sm) was 4.23 μg/mL for a 20% decrease in the Sm. The estimate of γ with RSE and interindividual variability presented as % coefficient variation was 4.3 (36.8, 12.5%) for a 20% decrease. Interindividual random variability was modeled using a log-normal model.RSE = relative standard error.
|




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download