Abstract
Boerhaave syndrome is a transmural perforation of the esophagus and typically occurs after forceful emesis. Boerhaave syndrome is a destructive disease with a high mortality rate, though surgical intervention within 24 hours has a beneficial effect. On the other hand, late surgical intervention is associated with poorer prognoses. Several therapeutic strategies, ranging from medical to surgical management, are available for Boerhaave syndrome. Recently, endoscopic endoluminal vacuum therapy (EVT) was introduced as a treatment option. Here, we report the case of a 56-year-old male patient with Boerhaave syndrome who was successfully treated by EVT after primary closure failure. The patient recovered without complication.
Boerhaave syndrome is a serious disease, and early diagnosis followed by primary closure is the best treatment.1 However primary closure has a high failure rate when performed more than 24 hours after symptom onset. Recently, vacuum therapy, which is well established for treatment of chronic surface wounds, was successfully applied to esophageal perforation.2 Here, we report our experience of managing a patient by endoscopic endoluminal vacuum therapy (EVT) after primary closure failure.
A 56-year-old man presented with a cough and epigastric pain that began 48 hours earlier after he had vomited after consuming alcohol. At presentation his blood pressure was 83/49 mmHg, heart rate 100 bpm, body temperature 36.7℃, white blood cell count 9,740/µL, CRP 33.6 mg/dL, and procalcitonin 39.3 ng/mL. Chest CT revealed esophageal rupture with accompanying pneumomediastinum and pleural effusion (Fig. 1A), and esophagogastroscopy (EGD) showed a 3 cm longitudinal perforation above the esophagogastric junction (Fig. 1B). Accordingly, Boerhaave syndrome was diagnosed. Emergency primary closure was performed via left posterolateral thoracotomy. An abscess was present in the left thoracic cavity and gross pus in the periesophageal space (Fig. 1C). Fever persisted until postoperative day (POD) 5 when white blood cell count was 17,590/µL and EGD showed perforation where the esophagus had been sutured. The perforation was confirmed in the operating room. Primary closure was reperformed using a viable remnant of esophageal mucosa and surrounding muscles. However, 6 days later on POD 11, fever persisted at 38.0℃ and EGD detected a hole at the suture site. Exploratory thoracotomy on POD 11 confirmed the perforation had not healed and showed perforated esophageal tissue was not viable for another primary closure. A T-tube was inserted into the perforated esophagus and feeding jejunostomy was performed on POD 11. After each operation, the patient was admitted to our intensive care unit for one day, but was otherwise treated in a general ward. The day after first primary closure, we started total parenteral nutrition and ensured enteral nutrition using a jejunostomy feeding catheter from POD 19. Although there was no evidence of inflammation (Fig. 2), routine EGD follow-up at week 4 confirmed persisting paraesophageal cavity (Fig. 3A, B). We decided to apply EVT to sterilize the space and achieve closure of the para-esophageal cavity.
EVT was performed using the commercially available CuraVac® system (BioAlpha Inc., Seongnam, Korea) (refer to Fig. 3C, D). A Levine tube (18 Fr.) was inserted through the nose to the mouth. A polyethylene sponge was cut to fit into the perforated cavity and sutured to the tip of the Levine tube (Fig. 4). An endoscope forceps was used to hold the sponge and pushed into the perforated cavity by an experienced endoscopist, and a vacuum pump was connected to provide continuous negative pressure (80-100 mmHg).
Regarding antibiotic administration during his hospital stay, empirical cefotaxime and metronidazole were administered from POD 2 to 8, vancomycin, meropenem, and fluconazole from POD 9 to 17, piperacillin/tazobactam, teicoplanin, and fluconazole from POD 18 to 49, and ampibactam, ciprofloxacin, and fluconazole (oral antibiotics) from POD 50 to 84, when antibiotics were discontinued. Antibiotics were administered in consultation with an expert in infection medicine.
Function of EVT was evaluated repetitively. When EVT conveyed lack of performance such as decreased suction capacity or evidence of blockage due to saliva accumulation around the sponge, change of EVT was carried out. Within the course of 5 weeks period, six EVT change was conducted. Duration of each EVT lasted from 4 days, 3 days, 6 days, 7 days and 7 days in sequential order, respectively. Wound healing progressed during this period as indicated by observed when sponges were changed, and during the last EVT it was evident that the cavity had been successfully closed (Fig. 5). Thirty-seven days after the first EVT (on POD 71), the patient started to drink water and 3 later was able to take a soft diet. He was discharged without further sequelae was followed at our outpatient clinic for 6 months during which he was on a tolerable diet and increased in weight.
Therapeutic strategies for Boerhaave syndrome range from medical treatment to surgical management, but despite recent progress in its treatment, mortality is high when treatment is initiated more than 24 hours after symptom onset.1 It has been well-established that surgical intervention to achieve primary closure within 24 hours of onset lowers mortality, and some have reported good outcomes for surgical repair conducted more than 24 hours after onset.13 In our case, the perforation occurred more than 24 hours before surgery, but fortunately, esophageal tissue remained relatively viable, and thus, primary closure was attempted.
If primary closure is unsuccessful or esophageal tissue is too friable to hold a suture, a controlled fistula could be created using a T-tube to control infection and facilitate healing.14 In our patient, primary closure failed, and T-tube insertion was performed to localize drainage and control infection. However, after inflammation had subsided, a cavity formed at the T-tube insertion site.
Vacuum therapy was first introduced for plastic and reconstructive surgery in 1997, and since has been increasingly to treat diverse wound infections.5 A literature search identified 12 case reports of patients with Boerhaave syndrome treated by EVT (Table 1). This novel endoscopic procedure was designed to close intestinal defects, and in appropriate cases, represents an effective alternative treatment for esophageal defects.6 As compared with conventional methods of drainage and treatment, vacuum therapy enhances patient comfort, but it should be noted that vacuum therapy cannot be regarded as a universal cure for every infected wound, and thus, classical surgical techniques constitute important components of the therapeutic armamentarium used for treating infected wounds.7 The described case was the first to be treated by EVT at Kyungpook National University Hospital, and it was noted that the third procedure conducted by the skilled endoscopist involved took less time.
Many treatment options are available for esophageal perforation, such as different surgical interventions, stents, endoscopic gluing, and clip devices. The goal of using an esophageal stent to cover an esophageal perforation is to prevent contamination and enable early ingestion. Small iatrogenic perforation is the primary indication for esophageal stenting, and its primary complication is stent migration. Esophageal stenting can be used to address small esophageal perforations and perforations diagnosed early, that is, before contamination of mediastinum. Another method to treat esophageal perforation is clipping through approximation of mucosa. However clipping can be only used on perforation of small sizes due to relatively small diameter of the clips (<11 mm).189 Endoscopic gluing was initially proposed for the management of esophageal fistula, but has rarely been reported for esophageal perforation.110 A recent study compared the results of EVT, endoscopic clipping, and esophageal stenting in cases of esophageal leakage after esophageal surgery, and found EVT achieved a higher wound closure rate (84.4%) than stenting (53.8%).11 In another recent study, in which EVT was adopted as the primary intervention and as a rescue modality for operative failure, a closure rate of 92% was achieved.2 Based on these wound closure rates, we decided to use EVT for infection control in our patient.
Vacuum therapy enables the perforated cavity to be cleaned and actively drained using a minimally invasive approach, and thus, reduces bacterial contamination and local edema and promotes granulation and wound healing.1213 In the described case, EGD was performed twice weekly in order to monitor the wound and enable rapid response to any negative changes. However, nasal tubes can cause significant discomfort, it is difficult to perform EGD frequently, and EGD lengthens recovery times after surgical treatment. In our patient, we used EGD to confirm EVT promoted wound cavity healing.
Drainage and nutrition are important aspects of infection control and esophageal healing. In our patient, a chest tube and a T-tube were used for external drainage and EVT for internal drainage. Initially the patient was supported by total parenteral nutrition, and on confirming healing was delayed, jejunostomy was performed and enteral feeding started. We believe this type of supportive care and EVT played important roles in our patient's recovery. We conclude EVT is a promising treatment option in Boerhaave syndrome when surgical treatment fails.
Figures and Tables
 | Fig. 1(A) Chest computed tomography image obtained at admission showing periesophageal pneumomediastinum (arrow) and empyema (arrowhead). (B) Esophagogastroscopy image showing the tear site and pus. (C) The perforated esophagus and pus found during surgery. |
 | Fig. 2Graph showing inflammatory marker trends after hospitalization. WBC, white blood cell; EVT, endoscopic endoluminal vacuum therapy; CRP, C-reactive protein. |
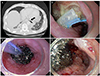 | Fig. 3(A) Chest computed tomography image obtained on day 18 of EVT shows a contained periesophageal pouch (arrow) without evidence of mediastinitis or empyema. (B) Esophagogastroscopy image showing a periesophageal pouch and inserted T-tube, (C) a polyethylene sponge sutured to the tip of the Levin tube, and (D) the polyethylene sponge delivered to the pouch. T, T-tube; L, Levin tube; S, sponge; EVT, endoscopic endoluminal vacuum therapy. |
 | Fig. 4Sponge preparation for endoscopic endoluminal vacuum therapy. (A) Sponge, Levin tube, needle holder, scissors, and needle. (B) The Levin tube was cut to remove proximal side hole, and the sponge was trimmed to fit into the perforated cavity and sutured to the tip of the tube. (C, D) A ring was prepared and held with the endoscope forceps and to able sponge delivery. |
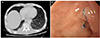 | Fig. 5The patient was discharged and followed in an outpatient clinic. (A) A comparison of follow-up chest computed tomography (CT) images obtained at 21 days after final endoscopic endoluminal vacuum therapy and previous CT images showed the periesophageal pouch had disappeared. (B) Esophagogastroscopy image at 6 months after final endoluminal vacuum therapy showing successful healing of the esophageal tear (arrow). |
References
1. Chirica M, Champault A, Dray X, et al. Esophageal perforations. J Visc Surg. 2010; 147:e117–e128.

2. Still S, Mencio M, Ontiveros E, Burdick J, Leeds SG. Primary and rescue endoluminal vacuum therapy in the management of esophageal perforations and leaks. Ann Thorac Cardiovasc Surg. 2018; 24:173–179.



3. Kim KD, Chung KY, Kim CS, Park HG. Delayed primary repair of esophageal rupture. Korean J Thorac Cardiovasc Surg. 1998; 31:46–51.
4. Do YW, Lee CY, Lee S, Kim HE, Kim BJ, Lee JG. Successful management of delayed esophageal rupture with T-tube drainage using video-assisted thoracoscopic surgery. Korean J Thorac Cardiovasc Surg. 2016; 49:478–480.



5. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997; 38:563–576.

6. Loske G, Schorsch T. Endoscopic vacuum therapy for Boerhaave’s syndrome. Chirurg. 2016; 87:676–682.

7. Listewnik MJ, Sielicki P, Mokrzycki K, Biskupski A, Brykczyński M. The use of vacuum-assisted closure in purulent complications and difficult-to-heal wounds in cardiac surgery. Adv Clin Exp Med. 2015; 24:643–650.


8. Johnsson E, Lundell L, Liedman B. Sealing of esophageal perforation or ruptures with expandable metallic stents: a prospective controlled study on treatment efficacy and limitations. Dis Esophagus. 2005; 18:262–266.


9. Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007; 66:605–611.


10. Rábago LR, Castro JL, Joya D, et al. Esophageal perforation and postoperative fistulae of the upper digestive tract treated endoscopically with the application of Tissucol. Gastroenterol Hepatol. 2000; 23:82–86.

11. Brangewitz M, Voigtländer T, Helfritz FA, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy. 2013; 45:433–438.


12. Heits N, Stapel L, Reichert B, et al. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg. 2014; 97:1029–1035.


13. Möschler O, Nies C, Mueller MK. Endoscopic vacuum therapy for esophageal perforations and leakages. Endosc Int Open. 2015; 3:E554–E558.

14. Bludau M, Hölscher AH, Herbold T, et al. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc. 2014; 28:896–901.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download