Abstract
Cystic neoplasms of the pancreas consist of a wide range of pathological entities and are being detected more frequently due to advances in cross-sectional imaging modalities and increasing numbers of periodic health checkups. The majority of pancreatic cystic neoplasms are intraductal papillary mucinous neoplasms, serous neoplasms, and mucinous cystic neoplasms, but recently, rare cases of mucinous non-neoplastic cyst of the pancreas (MNCP) have been reported, and despite the availabilities of modern imaging systems, such as MRI and CT, the differentiation of non-neoplastic and neoplastic cysts remains challenging. Herein, we report our experience of a 65-year-old male case with an MNCP.
Cystic neoplasms of the pancreas constitute of a wide range of pathologic entities and are the second-most common exocrine pancreatic neoplasm. Given advances in modern cross-sectional imaging techniques and a recent increase in the numbers of periodic health checkups, cystic lesions of the pancreas are now commonly detected. Furthermore, uncommon, distinct cystic pancreatic lesions, known as mucinous non-neoplastic cysts of the pancreas (MNCP), have been reported recently.1 We present the second reported case of MNCP in Korea.
A 65-year-old male was admitted for the evaluation of abnormal upper gastrointestinal endoscopy findings during a routine health checkup, namely, an external compressive lesion at the posterior wall of the stomach midbody (Fig. 1). The patient was admitted for evaluation of the lesion.
At admission, serum amylase and lipase levels were mildly elevated to 121 IU/L (normal range [NR], 28-100 IU/L) and 137 IU/L (NR, 13-60 IU/L). Tumor markers such as CA 19-9 and CEA were within normal limits. Clinical signs suggesting pancreatitis and other diseases were not observed. Routine laboratory results, including those of serological testing for hepatitis B and C, were negative. Initial CT demonstrated a single 2.5 cm-sized, round, hypoattenuated cystic lesion with no evidence of an enhanced solid portion in the pancreatic body (Fig. 2A), mild dilation of the main pancreatic duct, and delayed parenchyma enhancement of the pancreatic tail. Three-dimensional MRCP imaging depicted a 2.5 cm cystic nodule without a solid portion in the pancreas body with multiple, small, surrounding cystic nodules (Fig. 2B). Endoscopic ultrasound fine needle aspiration using Sonovue (Bracco Corp., Milano, Italy) was performed to differentiate serous and mucinous cysts, and Sonovue demonstrated a 2.5 cm sized, non-enhanced, slightly heterogenous, non-septated, serous cystic lesion with mural nodularity and mild pancreatic duct dilatation (Fig. 2C). Cytology and biopsy results of the FNA tissue revealed the presence of inflammatory cells, small numbers of benign ductal cells, histiocyte and fibrohistiocytic infiltrations and atrophic acinar glands. In addition, tumor marker levels were elevated in aspirated fluid CEA level 17,157 ng/dL (NR, 0–4.6 ng/dL) and CA 19-9 level was 121.6 U/mL (NR, 0-35 U/mL). Cystic fluid amylase and lipase levels were 27 and 75 IU/L, respectively. Based on the above the lesion was inferred to be a mucinous cystic neoplasm.
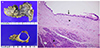
The patient underwent spleen-preserving distal pancreatectomy. Final histology demonstrated a single cyst lined by mucinous epithelium (Fig. 3A), and pathology found no evidence of a metaplastic, dysplastic, or malignant lesion. Immunohistochemically, epithelial cells were positive for MUC-1 but negative for MUC-2. Ki-67 was below 1%. Based on all results, the final diagnosis was MNCP (Fig. 3B).
Cystic neoplasms are a heterogeneous group of lesions and have been meticulously described by Klöppel and Kosmahl2 and classified as neoplastic or non-neoplastic and epithelial or non-epithelial. Neoplastic epithelial lesions are classified as benign, borderline, or malignant and are usually serous cystadenomas, mucinous cystic neoplasms (MCNs), or IPMNs. Neoplastic non-epithelial lesions are either lymphangiomas or sarcomas, and non-neoplastic epithelial cysts are either congenital, lymphoepithelial, retention, or endometrial cysts or MNCPs.1 Non-neoplastic non-epithelial cysts are serous cystadenomas accompanied by pseudocysts and parasitic cysts. Notably, many patients with a pancreatic cyst have non-neoplastic, inflammatory pseudocysts that develop as a complication of acute pancreatitis.3
Kosmahl et al.1 presented a novel non-neoplastic cystic lesion of the pancreas in five patients with MNCP in 2002. This benign cyst is lined with a monolayer of cuboidal to columnar mucinous epithelium and appears to be unilocular or multilocular. MNCPs have been reported to account for 2.1–3.4% of pancreatic lesions,45 over a wide age range (20 to 88 years) in both genders, but with a preference for women of over 50 years.145 MNCP usually affects the pancreatic head and presents as a single cyst (30% of patients present with multiple lesions). The clinical presentations of MNCP are usually non-specific and obstructive jaundice due to external compression of the common bile duct is rare.
Clinicopathologically, MNCP is characterized by mucinous differentiation of lining epithelium, lack of cellular atypia or increased proliferation, thin rim of almost acellular supporting stroma, the absence of communication with pancreatic ducts, and a preference for the pancreatic head.1 MNCPs do not exhibit any neoplastic features, such as dysplasia, proliferative activity, an invasive growth pattern, or metastatic spread. The origin and development of this entity remains speculative.5
MNCP must be differentiated from other cystic tumors of the pancreas lined with mucinous pancreatic epithelium, such as MCNs, IPMNs, and retention cysts. As regards the differentiation of MNCP and IPMN (the most common cystic pancreatic lesion), MNCP is not found within pancreatic ducts (intraductal), whereas MNCPs and IPMNs both are characterized by the production of thick mucinous fluid. MNCP also lacks the ability of IPMN to communicate with pancreatic duct and neoplastic cells. Retention cysts may occur secondary to intraluminal obstruction of the pancreatic duct from calculi, viscous mucin, and fibrosis caused by chronic pancreatitis or neoplastic infiltration of periductal tissue. Accordingly, retention cyst can be excluded by the absence of potential causes or evidence of ductal obstruction and the absence of communication between the cyst and the pancreatic duct.
MCNs are mucin-producing cystic tumors that lack communication with the pancreatic duct and contain mucin-producing columnar epithelium. Ovarian-like stroma surrounding columnar epithelium is considered to be pathognomic, and although MNCPs and MCNs have similar epithelial phenotypes, their stromal components and potentials for malignant transformation differ.6
In our patient, MRI revealed a 2.5 cm, unilocular, macrocystic lesion with a smooth cystic wall without intramural septation or mural nodularity in the body portion of the pancreas. Endoscopic ultrasound demonstrated a 2.5 cm cystic lesion without septum or nodules. Interestingly, the cyst was found to contain mucinous fluid with a hugely increased CEA level (17,157 ng/dL; NR, 0–4.6 ng/dL). Brugge et al.7 concluded CEA levels in cystic fluid aid the diagnosis of mucinous cysts, and that a cutoff value of 192 ng/mL was 80% accurate. In another study, the presence of mucin and an elevated CEA concentration in cystic fluid was found to have a sensitivity and specificity of 73% and 65%, respectively, for identifying mucinous cysts.8 However, CEA concentration has not been shown to the ability to predict the presence of invasive disease. Notably, CEA levels in serous cystadenomas and pancreatic pseudocysts are undetectable.9
MNCPs are known to be benign,145 and our patient remained in good health with no evidence of recurrence at her 12-month follow-up. A large-scale cohort study is needed to provide guidance on the accurate diagnosis and prognosis of MNCP because only a few small-scale studies have been conducted to date.
Figures and Tables
 | Fig. 1Upper gastrointestinal endoscopy revealed an external compressive lesion at the posterior wall of the stomach midbody. |
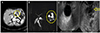 | Fig. 2(A) Abdominal computed tomography image showing a 2.5 cm, round, hypoattenuated cystic lesion without any enhanced solid portion in the pancreatic body. (B) Three-dimensional magnetic resonance cholangiopancreatogram showing a 2.5 cm, cystic mass without any solid portion in the pancreatic body surrounded by multiple, small daughter cysts. (C) Endoscopic ultrasound revealed a non-septated, 2.5 cm, single cyst with mural nodularity. Circles and an arrow indicated a cystic lesion. |
References
1. Kosmahl M, Egawa N, Schröder S, Carneiro F, Lüttges J, Klöppel G. Mucinous nonneoplastic cyst of the pancreas: a novel nonneoplastic cystic change? Mod Pathol. 2002; 15:154–158.


2. Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology. 2001; 1:648–655.

4. Cao W, Adley BP, Liao J, et al. Mucinous nonneoplastic cyst of the pancreas: apomucin phenotype distinguishes this entity from intraductal papillary mucinous neoplasm. Hum Pathol. 2010; 41:513–521.


5. Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreasand tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004; 445:168–178.

6. Yang JD, Song JS, Noh SJ, Moon WS. Mucinous non-neoplastic cyst of the pancreas. Korean J pathol. 2013; 47:188–190.

7. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004; 351:1218–1226.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download