Abstract
Anaerobic infections have been reported to be responsible for 3–10% of pyogenic liver abscesses in Korea, and reported anaerobes include Fusobacterium, Bacillus fragilis, and Bacteroides melaninogenicus. Parvimonas micra is an anaerobic, Gram-positive, non-spore-forming bacterial species and a constituent of normal flora on skin, vagina, gastrointestinal tract, and oral cavity that can cause opportunistic infections. However, it has only rarely been reported to be a cause of liver abscess; only one such case has been reported in Korea. We experienced a case of concomitant liver and brain abscesses caused by Parvimonas micra in a non-immunodeficient 65-year-old female patient without diabetes or periodontal disease. Parvimonas micra infection was confirmed by blood culture using VITEK® 2 cards and by bacterial 16s ribosomal RNA gene sequencing. We conclude that we should not overlook anaerobes as a cause of liver abscess.
Anaerobes have been reported to be responsible for up to 10% of pyogenic liver abscesses in Korea,123 and the increasing incidence of such abscesses might be due to improved culture techniques or increased transportation. Parvimonas micra (P. micra) is an anaerobic component of normal flora of skin, vagina, gastrointestinal tract, and oral cavity, and a known, but rare, cause of liver and brain abscess. However, no case of concomitant liver and brain abscesses has been previously reported. Here, we describe our experience of a case of concomitant liver and brain abscesses caused by P. micra.
A 65-year-old woman was hospitalized due to altered mentality. The patient lived alone and had a 2- to 3-week history of poor oral intake, nausea, and weakness. She had been found unconscious and brought to emergency room of Dong-A University Hospital by her sister. She had no particular underlying disease, including periodontal disease, but had poor hygiene and 5 cm sized, grade II pressure sore of the coccyx. At admission, she had drowsy mentality and acutely ill-looking appearance. On examination, she had a sinus tachycardia (113 beats per minute), blood pressure 100/70 mmHg, body temperature 37.1℃, and a blood glucose level of 190 mg/dL. Some crackles were detected during chest auscultation at the right lung base. Heart sounds and abdominal examination were normal. She had dehydrated tongue but no neck stiffness. Her Glasgow coma scale (GCS) score was 9, although accurate examination was difficult due to a drowsy mentality and general weakness. Neurologic examination showed motor, sensory system, and reflexes were relatively intact.
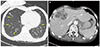
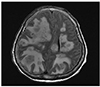
Laboratory results were as follows; white blood cell 33,920/mm3 (neutrophils 91%), hemoglobin 10.6 g/dL, platelet 38,000/mm3, CRP 15.6 mg/dL, procalcitonin 29.8 ng/mL, total bilirubin 1.9 mg/dL, direct bilirubin 0.6 mg/dL, BUN 43 mg/dL, creatinine 0.73 mg/dL, INR 1.21, and ammonia 45 umol/L. Other laboratory findings including AST, ALT, ALP levels were within normal reference ranges. Chest X-ray showed multifocal consolidation and subsegmental atelectasis. Brain CT without contrast demonstrated multifocal hypodense nodular legions in bilateral cerebral hemispheres and cerebellum. Brain MRI with contrast revealed multiple, rim enhanced nodules in whole brain consistent with abscesses but without any ventricular abnormalities (Fig. 1). Chest CT revealed multiple septic embolisms (Fig. 2A) and a CT scan of the liver showed an immature abscess with a honeycomb appearance in the S4/8 area (Fig. 2B) but no definite primary infection focus in other organs (genitourinary, gastrointestinal tract, etc.).
Blood and pressure sore cultures were performed. Lumbar puncture showed polydominant white blood cell (360/mm3) and an elevated protein level (135 mg/dL) in cerebrospinal fluid. Empirical cefotaxime and metronidazole antibiotic therapy was started. No organism was identified by pressure sore or cerebrospinal fluid culture. However, blood culture under anaerobic conditions showed the presence of gram-positive bacterial colony, which was subsequently identified as P. micra using the VITEK® 2 microbial identification system (BioMérieux, Marcy-l'Etoile, France) and by bacterial 16s ribosomal RNA (rRNA) gene sequencing using previously described primers (5′-GGATTAGAT ACCCTGGTA-3′ [785F], 5′-CCGTCAATT CMTTTRAGTTT-3′ [907R]) (percentage match 99%). The patient was referred to the dental medicine for searching of the infection focus, but she had not dental disease including periodontitis. And transthoracic echocardiogram did not show evidence of endocarditis. Four days after admission, follow up blood cultures showed no bacterial growth, and at 7 days after admission, follow up abdominal ultrasound showed the size of liver abscess had not changed, and thus, aspiration was performed. Culture of the pus was negative for microorganisms. Despite antibiotic therapy and improved laboratory findings, her consciousness worsened. On admission day 14, abdominal ultrasound showed the liver abscess had diminished in size, but follow up brain MRI revealed aggravation of multiple brain abscesses and brain edema with midline shift (Fig. 3). Treatment with antibiotics and with dexamethasone and mannitol for cerebral edema her condition deteriorated. The patient's family then refused further treatment due to financial difficulties and she was discharged for hospice care.
Pyogenic liver abscesses are the result of bacterial infections of liver parenchyma and are usually caused by Klebsiella pneumoniae or Escherichia coli in Korea.123 The prevalence of anaerobic infection among culture (blood or non-blood) positive pyogenic liver abscess patients has been reported to be 20–30% in western countries45 and 3–10% in Korea,123 and recently, it has been more frequently reported. Reported anaerobes isolated include Fusobacterium, Bacillus fragilis and Bacteroides melaninogenicus.67
Only one previous case of pyogenic liver abscess caused by P. micra has been reported in Korea,3 but concomitant liver and brain abscesses caused by P. micra have never been previously reported. We present a case of liver and brain abscesses caused by P. micra isolated from a blood sample. The increased incidence of anaerobic infection is probably due to advanced culture techniques and the availability of 16s rRNA sequencing. Several commercial kits for the identification of anaerobic isolates are now available. These include the VITEK 2 ANI card, the RapidID 32A system, the API 20A system, and the BBL crystal ANR ID kit.8 In the present study, we also performed 16s rRNA sequencing for to ensure accurate identification of the pathogen. This sequencing technique is useful for identifying bacteria strains with unusual phenotypes or that are slow growing, rare, or uncultivable using conventional methods and helps clinicians choose suitable antibiotics.9
P. micra (former name Peptostreptococcus micros, Micromonas micros) is a species of anaerobic Gram-positive cocci, which is widely distributed on skin, vagina, gastrointestinal tract, and oral cavity, and able to cause opportunistic infections.10 Cobo et al.11 recently had reviewed 30 cases of P. micra infection in the literature and provided sufficient details to enable comparisons. According to this review, the spine is the preferred site of infection (45.1%), followed by joints, heart valves, and pleura.11 Reported common risk factors of P. micra infection include dental conditions (e.g., periodontitis, dental carries, tooth extraction), poor oral health, immunodeficiency and diabetes mellitus.1112
Although in our patient medical history details were inadequate due to decreased mentality, there was no evidence of periodontitis or tooth extraction by physical examination or CT, but oral hygiene was poor and a pressure sore was present on her coccyx. It is known simple chewing and brushing teeth can induce spontaneous bacteremia, particularly in subjects with poor oral health, and that normally transient bacteremia can cause systemic disease in those with a weakened defense mechanism due to a immunosuppression disorder or malnutrition.12 Furthermore, P. micra has been reported to be the primary infectious agent in 4% of chronic pressure ulcers.13 Therefore, we suggest bacteremia from the oral cavity or from a skin infection accessed the arterial circulation and hepatic arterial tree and caused the hepatic abscess in our patient.
Infections by anaerobes are generally treated with broad spectrum antibiotics, but because of the complexities of susceptibility tests and the low rate of antibiotic resistance to anaerobes, tests are not performed routinely. Previous studies have reported P. micra is usually susceptible to metronidazole, penicillin, amoxicillin, amoxicillin-clavulanic acid, piperacillin, piperacillin-tazobactam, cefoxitin, imipenem, and ciprofloxacin,14 but rare resistance to metronidazole has been reported recently and the treatment of choice for P. micra infections has not been established.11 We initially treated our patient with cefotaxime and metronidazole based on the considered likelihood of a polymicrobial infection, and maintained this regimen after P. micra has been identified. Susceptibility testing was not performed because of technical limitations.
Although the liver abscess showed partial resolution after antibiotic treatment, multiple brain abscesses and brain edema were gradually aggravated. Aspiration, long-term treatment, and susceptibility testing are required in this situation.
Concomitant liver and brain abscesses are uncommon, but according to previous articles on the subject, long-term (1–4 months) antibiotic treatment results in effective resolution and has a favorable prognosis.15161718 Poor prognostic factors for brain abscess included a low mean GCS score at presentation, and the presence of septic shock and neck stiffness.19 Our patient had a low GCS score (9) and septic shock at presentation and unfortunately a poor outcome.
Anaerobic liver abscess has been reported to be uncommon in Korea, and thus, there is a risk of overlooking the possibility of an anaerobic etiology. Accordingly, it is important to choose adequate broad-spectrum antibiotics for initial treatment and to identify the pathogen responsible rapidly and reliably to achieve early, accurate diagnosis and ensure effective treatment. Thus, we emphasize the importance of culture for both aerobes and anaerobes and the use of tools like bacterial 16s rRNA PCR amplification and sequencing to identify bacteria responsible.
Figures and Tables
 | Fig. 1Axial contrast-enhanced T2-weighted magnetic resonance imaging taken at admission showing multifocal brain abscesses. |
References
1. Chung DR, Lee SS, Lee HR, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect. 2007; 54:578–583.


2. Kim JK, Chung DR, Wie SH, Yoo JH, Park SW. Korean Study Group for Liver Abscess. Risk factor analysis of invasive liver abscess caused by the K1 serotype Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 2009; 28:109–111.


3. Ha J, Choi SP, Lee WH, et al. A clinical study on pyogenic liver abscesses: the changes in the clinical features during the recent 12 years. Korean J Med. 2008; 74:37–50.
4. Branum GD, Tyson GS, Branum MA, Meyers WC. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg. 1990; 212:655–662.



5. Seeto RK, Rockey DC. Pyogenic liver abscess. Changes in etiology, management, and outcome. Medicine (Baltimore). 1996; 75:99–113.


6. Sabbaj J, Sutter VL, Finegold SM. Anaerobic pyogenic liver abscess. Ann Intern Med. 1972; 77:627–638.


7. Kim YH, Yoon HJ, Park CW, et al. A case of liver abscess caused by Fusobacterium nucleatum in a patient with recurrent periodontal diseases. Korean J Gastroenterol. 2011; 57:42–46.

8. Lee EH, Degener JE, Welling GW, Veloo AC. Evaluation of the vitek 2 ANC card for identification of clinical isolates of anaerobic bacteria. J Clin Microbiol. 2011; 49:1745–1749.



9. Woo PC, Lau SK, Teng JL, Tse H, Yuen KY. Then and now: use of 16s rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008; 14:908–934.


10. Murdoch DA, Shah HN. Reclassification of peptostreptococcus magnus (prevot 1933) holdeman and moore 1972 as finegoldia magna comb. nov. and peptostreptococcus micros (prevot 1933) smith 1957 as micromonas micros comb. nov. Anaerobe. 1999; 5:555–559.

11. Cobo F, Rodríguez-Granger J, Sampedro A, Aliaga-Martínez L, Navarro-Marí JM. Pleural effusion due to Parvimonas micra. A case report and a literature review of 30 cases. Rev Esp Quimioter. 2017; 30:285–292.

12. Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000; 2:897–906.


13. Dana AN, Bauman WA. Bacteriology of pressure ulcers in individuals with spinal cord injury: what we know and what we should know. J Spinal Cord Med. 2015; 38:147–160.



14. Lee Y, Park Y, Kim MS, et al. Antimicrobial susceptibility patterns for recent clinical isolates of anaerobic bacteria in South Korea. Antimicrob Agents Chemother. 2010; 54:3993–3997.



15. Kim DS, Kim HJ, Park KS, et al. A case of diabetic ketoacidosis precipitated by Klebsiella liver abscess and brain abscess. Korean J Med. 2011; 81:652–656.
16. Wagner KW, Schön R, Schumacher M, Schmelzeisen R, Schulze D. Case report: brain and liver abscesses caused by oral infection with Streptococcus intermedius. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:e21–e23.

17. Saeed S, Zafar U, Johnson LB. Fusobacterium causing concomitant brain and liver abscesses. Infect Dis Clin Pract. 2005; 13:265–267.

18. Chen D, Dong M, Zhao K, Sun F, Wang H, Liu Z. Unusual synchronous liver and brain abscesses infected by rare Aerococcus viridians in a patient with pulmonary arteriovenous malformations on FDG PET/CT: a case report and literature review. Medicine (Baltimore). 2017; 96:e9048.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download