This article has been
cited by other articles in ScienceCentral.
Abstract
Orthostatic hypotension (OH) is commonly associated with autonomic failure in the peripheral nervous system. Less often it is related to central lesions in brainstem and cerebellum. We describe a patient with OH associated with tuberculosis meningoencephalitis involving the brainstem including rostral ventrolateral medulla. This is the first case of OH resulting from focal lesions in the dorsal medulla in a patient with meningoencephalitis.
Keywords: Orthostatic hypotension, Rostral ventrolateral medulla, Meningoencephalitis
Orthostatic hypotension (OH) is defined as a decrease in systolic blood pressure (BP) of at least 20 mmHg or diastolic BP of at least 10 mmHg within 3 minutes after standing.
1 OH occurs in up to 30% of the elderly and increases with age. The most common symptoms of OH are dizziness, light-headedness, palpitations, tremulousness, anxiety and nausea. In elderly patients, these symptoms can seriously impair quality of life and contribute to increased mortality and morbidity, causing falls and thus multiple fractures, as well as head injury. OH is related to several lesions at different levels in both the peripheral (PNS) and central (CNS) nervous systems,
2 but is associated more frequently with gradual failure of the autonomic PNS than with CNS lesions.
3 However, OH may also arise from destructive focal lesions involving the brainstem, spinal cord and cerebellum.
45678 Here, we describe a patient with OH associated with tuberculosis meningoencephalitis involving the brainstem including rostral ventrolateral medulla (RVLM).
CASE
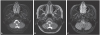
A 37-year-old female was admitted for symptoms of general malaise, fever, severe headache and vomiting for 7 days. She had no history of hypertension, diabetes, hepatitis or tuberculosis. After admission, she complained of refractory severe headache and a high spiking fever, but her neurologic examination was normal except mild neck stiffness. Bruzinski sign and Kernig sign were not shown. The blood laboratory findings were also normal, except for a mildly elevated CRP level (0.86 mg/dL). A cerebrospinal fluid (CSF) analysis revealed a white blood cell count of 83/uL, predominated by lymphocytes, and a high mTP (protein) of 157.7 mg/dL, but otherwise was normal. Acid-fast bacilli stain was negative, but the level of CSF adenosine deaminase (ADA) was slightly increased (6.6 U/L). Based on magnetic resonance imaging (MRI) showing leptomeningeal enhancement without parenchymal changes (
Fig. 1A), the patient was diagnosed with viral meningitis and treated conservatively, by hydration and symptomatic medications, under close observation. However, 4 days after admission, her mental status became drowsy, accompanied by upgaze deviation with upbeating nystagmus. On a repeat CSF analysis, white blood cell count was 63/uL, but ADA had increased markedly from 6.6 U/L to 13.6 U/L. Follow-up MRI showed aggravated leptomeningeal enhancement in the sulci of both cerebral hemispheres, especially around the midbrain and brainstem, and subtle T2 change in the medulla oblongata. The diagnosis of the patient was changed to CNS tuberculosis, and she was started on isoniazid, rifampin, ethambutol, pyrazinamide, and pyridoxine with intravenous dexamethasone. Ten days later, her mental status had recovered, but she had orthostatic dizziness with faintness and severe limb and trunk ataxia. A head-up tilt test showed a decrease in systolic BP of up to 60 mmHg immediately after standing without compensatory heart rate (HR) response (75 beats/min, 68 beats/min). A BP decrease of −30 mmHg persisted during the entire period of standing, consistent with classic OH. An autonomic function test showed the absence of a sympathetic skin response (SSR) (
Fig. 2A). HR responses to the Valsalva maneuver showed a transient decrease during first few seconds by activation of baroreceptors, but not followed by compensatory tachycardia (
Fig. 2B). Moreover, it showed an impaired late phase II response and increased pressure recovery time. According to SSR and Valsalva maneuver test, we noticed her sympathetic failure. A third MRI scan showed aggravated involvement of the medullary lesion, including the rostal ventrolateral medulla (RVLM) and both cerebellar hemispheres (
Fig. 1B, C). The patient was started on 0.5 mg midodrine and 0.2 mg fludrocortisone per day. One month later, the follow up examination showed mild ataxia and slightly improved OH, from a BP of 140/90 in supine position to 100/55 in the standing position. Three months later, the ataxia and OH had nearly resolved, and the medullary and cerebellar parenchymal changes had improved without scarring.
DISCUSSION
This is the first reported case of OH resulting from focal lesions in the dorsal medulla in a patient with meningoencephalitis. In previous studies, OH was associated with destructive focal lesions arising from a tumors, stroke, syringobulbia, or multiple sclerosis, involving the brainstem, spinal cord and cerebellum.
568
The maintenance of normal BP in the upright position depends on the integration of baroreflex and several important vascular beds, including those of striatal muscle, splanchnic-mesenteric, and cerebrovascular function. Two important baroreflexes are the vagal baroreflex pathway, from the nucleus tractus solitaruis (NTS) to the sinoartial node, and the adrenergic baroreflex pathway, from NTS to RVLM. The adrenergic pathway continues with sympathetic efferents from the RVLM to the intermediolateral thoracic spinal cord, autonomic ganglia and heart. OH is associated with impairment or the baroreflex sympathetically mediating the vasoconstriction of muscle and splanchnic vessels in response to a change in orthostatic position. Although the precise lesion resulting in OH has not been identified, lesions of the brainstem including the RVLM have been implicated. The RVLM contains the catecholaminergic transtegmental tract, which includes sympathoexcitatory glutamatergic neurons providing tonic excitatory input to sympathetic preganglionic neurons. This pathway is the critical efferent pathway for baroreflex-mediated vasoconstriction induced by orthostatic stress.
910 Idiaquez et al.
6 provided anatomical evidence supporting a relationship between the RVLM and OH. In their study, the patient had marked OH after undergoing partial removal of a cavernous angioma in a restricted medullary lesion involving the RVLM. Biopsy demonstrated that the resected lesion contained the catecholaminergic transtegmental tract arising from sympathoexcitatory neurons. In another case of OH attributed to a lower brainstem glioma, although the previous focal lesion was not identified, the damage included the lateral medullary vasomotor center adjacent to the RVLM.
3
Similar to previous case, our patient showed definite OH associated with a lesion in the lateral medulla related to tuberculosis. Although autonomic function test could not definite differentiate between central and peripheral autonomic failure and the exact nature of the anatomical lesion was not investigated by biopsy, this case demonstrates that OH may be triggered by interruption of the sympathoexcitatory pathway in the RVLM.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download