Abstract
Background
Sex influences is important to understand behavioral manifestations in a large number of neuropsychiatric disorders. We found electrophysiological differences specifically related to the influence of sex on psychopathic traits.
Methods
The resting electroencephalography (EEG) activity and low-resolution brain electromagnetic tomography (LORETA) for the EEG spectral bands were evaluated in 38 teenagers with conduct disorder (CD). The 25 male and 13 female subjects had psychopathic traits as diagnosed using the Antisocial Process Screening Device. All of the included adolescents were assessed using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria. The visually inspected EEG characteristics and the use of frequency-domain quantitative analysis techniques are described.
Results
Quantitative EEG (QEEG) analysis showed that the slow-wave activities in the right frontal and left central regions were higher and the alpha-band powers in the left central and bitemporal regions were lower in the male than the female psychopathic traits group. The current source density showed increases in paralimbic areas at 2.73 Hz and decreases in the frontoparietal area at 9.37 Hz in male psychopathics relative to female psychopathics.
Go to : 
References
2. Afifi M. Gender differences in mental health. Singapore Med J. 2007; 48:385–391.
3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed.Arlington: American Psychiatric Association;2013.
4. Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: gender differences and clinical variability. Am J Psychiatry. 2002; 159:934–940.

5. N⊘vik TS, Hervas A, Ralston SJ, Dalsgaard S, Rodrigues Pereira R, et al. Influence of gender on attention-deficit/hyperactivity disorder in Europe-ADORE. Eur Child Adolesc Psychiatry. 2006; 15(Suppl 1):I15–I24.
6. Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry. 2000; 39:1468–1484.

7. Munkvold LH, Lundervold AJ, Manger T. Oppositional defiant disorder gender differences in co-occurring symptoms of mental health problems in a general population of children. J Abnorm Child Psychol. 2011; 39:577–587.
8. Forouzan E, Cooke DJ. Figuring out la femme fatale: conceptual and assessment issues concerning psychopathy in females. Behav Sci Law. 2005; 23:765–778.
9. Cale EM, Lilienfeld SO. Sex differences in psychopathy and antisocial personality disorder. A review and integration. Clin Psychol Rev. 2002; 22:1179–1207.
10. Forth AE, Hart SD, Hare RD. Assessment of psychopathy in male young offenders. Psychol Assess. 1990; 2:342.

11. Forth AE, Mailloux DL. Psychopathy in youth: what do we know? In:. CB Gacono, editor. The clinical and forensic assessment of psychopathy: a practitioner's guide. 1st ed.Mahwah: Lawrence Erlbaum Associates;2000. p. 25–54.
12. Frick PJ, Bodin SD, Barry CT. Psychopathic traits and conduct problems in community and clinic-referred samples of children: further development of the psychopathy screening device. Psychol Assess. 2000; 12:382–393.

13. Horita M, Takizawa Y, Wada Y, Futamata H, Hashimoto T. Sex differences in EEG background activity: a study with quantitative analysis in normal adults. Rinsho Byori. 1995; 43:177–180.
14. Wada Y, Takizawa Y, Jiang ZY, Yamaguchi N. Gender differences in quantitative EEG at rest and during photic stimulation in normal young adults. Clin Electroencephalogr. 1994; 25:81–85.

15. Jung HM, Lee YS, Kim S, Kim SK, Jeong JS, Oh JS, et al. Sex-related differences of EEG coherences between patients with schizophrenia and healthy controls. J Korean Biol Pych. 2013; 20:166–178.
16. Langrová J, Kremláček J, Kuba M, Kubová Z, Szanyi J. Gender impact on electrophysiological activity of the brain. Physiol res. 2012; 61(Suppl 2):S119–S127.

17. Baving L, Laucht M, Schmidt MH. Oppositional children differ from healthy children in frontal brain activation. J Abnorm Child Psychol. 2000; 28:267–275.
18. Hermens DF, Kohn MR, Clarke SD, Gordon E, Williams LM. Sex differences in adolescent ADHD: findings from concurrent EEG and EDA. Clin Neurophysiol. 2005; 116:1455–1463.

19. Calzada-Reyes A, Alvarez-Amador A, Galán-García L, Valdés-Sosa M. QEEG and LORETA in teenagers with conduct disorder and psychopathic traits. Clin EEG Neurosci. 2017; 48:189–199.

20. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed.Washington: American Psychiatric Association;2000.
21. Frick PJ, Hare RD. Antisocial process screening device: APSD. 1st ed.Toronto: Multi-Health Systems;2001.
22. Hare RD. The psychopathy checklist–revised. 1st ed.Toronto: Multi-Health Systems;1991.
23. Ferrero RG, Ferreri AR. Computed EEG analysis. 1st ed.Buenos Aires: Las Heras;2006. p. 1–15.
24. Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994; 18:49–65.

25. Szava S, Valdes P, Biscay R, Galan L, Bosch J, Clark I, et al. High resolution quantitative EEG analysis. Brain Topogr. 1994; 6:211–219.

26. Galán L, Biscay R, Rodríguez JL, Pérez-Abalo MC, Rodríguez R. Testing topographic differences between events related brain potentials by using non-parametric combinations of permutation tests. Electroencephalogr Clin Neurophysiol. 1997; 102:240–247.
27. Pesarin F, Salmaso L. Permutation tests for complex data: theory, applications and software. 1st ed.Hoboken: John Wiley & Sons;2010. p. 25–45.
28. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002; 15:870–878.

29. Bennett WG, Korein J, Kalmijn M, Grega DM, Campbell M. Electroencephalogram and treatment of hospitalized aggressive children with haloperidol or lithium. Biol Psychiatry. 1983; 18:1427–1440.
30. Selin CL, Gottschalk LA. Schizophrenia, conduct disorder and depressive disorder: neuropsychological, speech sample and EEG results. Percept Mot Skills. 1983; 57:427–444.

31. Shaheen OO, Abdel Rahman S, Madkour O, Ahmed S. Conduct disorder in Egyptian children. Thesis submitted in partial fulfillment of requirements for the degree of M.D. 1990.
32. Tsuboi T. Seizures of childhood a population based and clinic based study. Acta Neurol Scand. 1986. (Suppl 110):1–237.
33. Clarke A, Barry R, McCarthy R, Selikowitz M. Age and sex effects in the EEG: differences in two subtypes of attention-deficit/hy-peractivity disorder. Clin Neurophysiol. 2001; 112:815–826.

34. Calzada-Reyes A, Alvarez-Amador A, Galán-García L, Valdés Sosa M. EEG abnormalities in psychopath and non-psychopath violent offenders. J Forensic Leg Med. 2013; 20:19–26.

35. Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012; 36:677–695.

36. Rangaswamy M, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, et al. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003; 27:607–615.

37. Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on P300 amplitude and topography in male adolescents. J Am Acad Child Adolesc Psychiatry. 2003; 42:106–115.

38. Éismont EV, Pritchenko OV, Pavlenko VB. Peculiarities of the pattern of EEG activity in institutionalized children. Neurophysiology. 2014; 46:415–442.

39. Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M. Magnetoen-cephalographic cortical rhythms. Int J Psychophysiol. 1997; 26:51–62.

40. Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry. 2011; 168:624–633.

41. Fairchild G, Toschi N, Hagan CC, Goodyer IM, Calder AJ, Passamonti L. Cortical thickness, surface area, and folding alterations in male youths with conduct disorder and varying levels of callous-unemotional traits. NeuroImage Clin. 2015; 8:253–260.

42. Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. J Am Acad Child Adolesc Psychiatry. 2013; 52:94–103.

43. Blair RJR. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci. 2008; 363:2557–2565.

44. Thatcher RW, Biver CJ, North D. Spatial-temporal current source correlations and cortical connectivity. Clin EEG Neurosci. 2007; 38:35–48.

45. Sheng T, Gheytanchi A, Aziz-Zadeh L. Default network deactivations are correlated with psychopathic personality traits. PLoS One. 2010; 5:e12611.

46. Juárez M, Kiehl KA, Calhoun VD. Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Hum Brain Map. 2013; 34:1921–1930.

47. Gao Y, Glenn AL, Schug RA, Yang Y, Raine A. The neurobiology of psychopathy: a neurodevelopmental perspective. Can J Psychiatry. 2009; 54:813–823.

48. Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev Psychopathol. 2005; 17:865–891.

49. Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006; 142:107–128.

50. Müller JL, Sommer M, Wagner V, Lange K, Taschler H, Röder CH, et al. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biol Psychiatry. 2003; 54:152–162.
51. Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001; 50:677–684.

Go to : 
 | Fig. 1.The anatomical distribution of the sources of delta-band activity (2.73 Hz) that were increased in the male psychopathic traits group were localized over a broad region within both hemispheres. The main regions where the activity was higher in the male than the female psychopathic traits group were the orbitofrontal cortex, supplementary motor area, fusiform area, rectus area, olfactory area, anterior cingulate, medial cingulate, medial temporal pole, parahippocampus, and amygdala in both hemispheres; the insula, superior temporal pole, medial temporal gyrus, and superior frontal gyrus in the right hemisphere; and the hippocampus in the left hemisphere. R, right hemisphere; L, left hemisphere; Z, the z-axis value for each image of the shown brain template. |
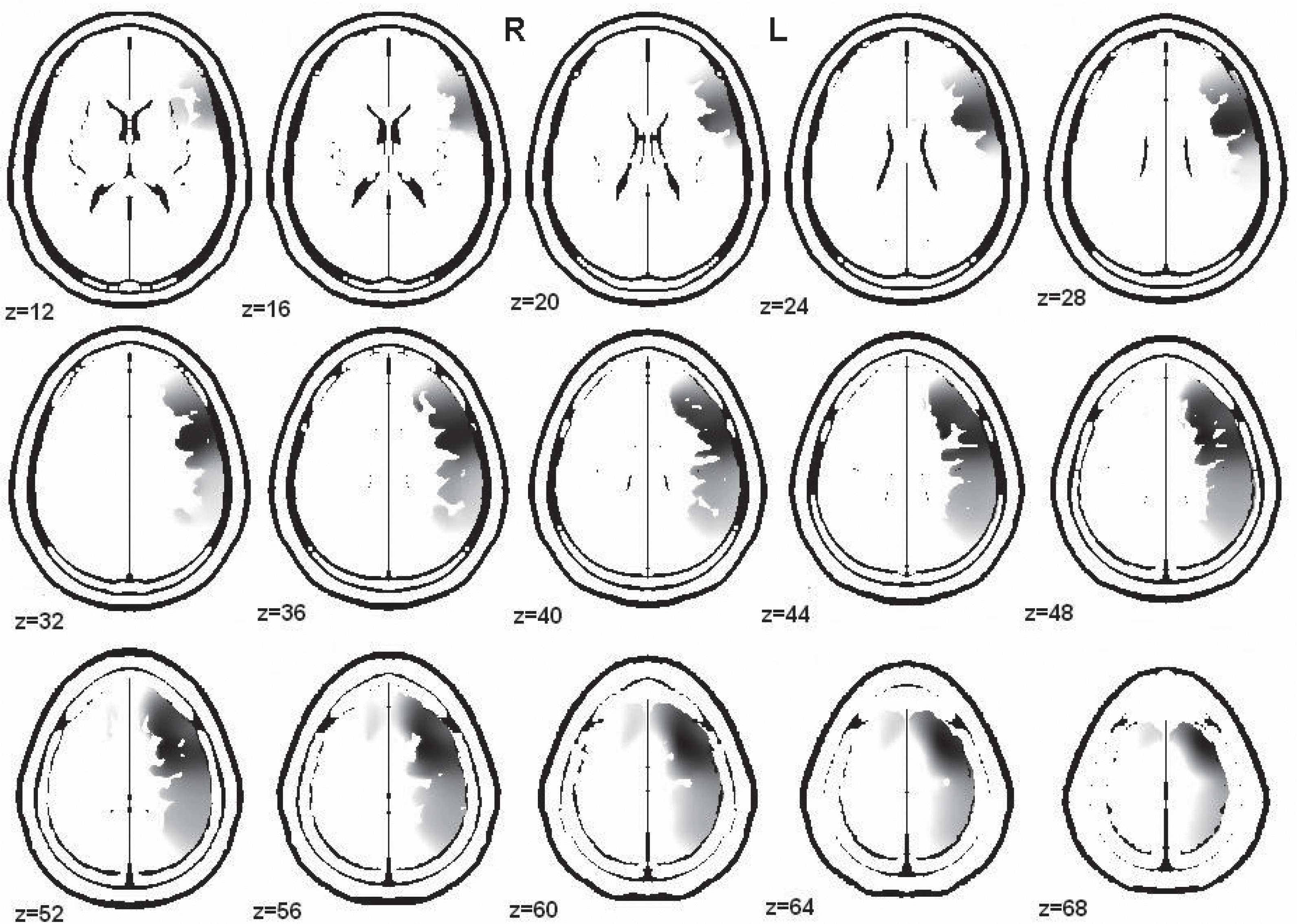 | Fig. 2.The anatomical distribution of the sources of alpha-band activity (9.36 Hz) that were decreased in the male psychopathic traits group were localized over a broad region within the left hemisphere. The main regions where the activity was lower in the male than the female psychopathic traits group were the orbitofrontal cortex, three parts of the inferior frontal gyrus, medial frontal gyrus, opercular parts of the inferior frontal gyrus, superior frontal gyrus, insula, precentral area, supplementary motor area, postcentral area, supramarginal area, superior parietal gyrus, inferior parietal gyrus, and angular gyrus in the left hemisphere. R, right hemisphere; L, left hemisphere; Z, the z-axis value for each image of the shown brain template. |
Table 1.
Comparison of demographic and behavior variables between the male and female teenagers with psychopathic traits
| Variable | Male psychopathic traits | Female psychopathic traits | Statistic |
|---|---|---|---|
| Age (years) | 15.66 (1.21) | 15.35 (0.66) | F = 0.40 |
| ASPD score | 27.76 (4.30) | 22.16 (6.09) | x2 = 0.00a |
| ADHD | 11 | 5 | x2 = 0.41 |
| Dissocial conduct disorder | 16 | 9 | x2 = 0.76 |
| Oppositional defiant disorder | 5 | 3 | x2 = 0.83 |
Table 2.
Classification of the adolescent electroencephalograms (EEG) in both groups by visual inspection
| Group | Normal | Slow | paroxysmal | Slow and paroxysmal |
|---|---|---|---|---|
| Male psychopathic traits | 1 (4.0) | 9 (36.0) | 12 (48.0) | 3 (12.0) |
| Female psychopathic traits | 3 (23.1) | 5 (38.5) | 4 (30.8) | 1 (7.7) |
Table 3.
Topographic distribution of electroencephalography (EEG) abnormalities
Table 4.
Brain areas showing significant differences (q = 0.2) within the delta band (2.73 Hz) between male and female teenagers with psychopathic traits according to a voxel-by-voxel analysis using low-resolution brain electromagnetic tomography (LORETA)
Table 5.
Brain areas showing statistical differences (q = 0.1) in alpha frequency (9.36 Hz) between male and female teenagers with psychopathic traits
According to voxel by voxel analysis with Low-Resolution Brain Electromagnetic Tomography (LORETA). Coordinates are given in millimeters, and the origin is at the anterior commissure. For x, negative values represent left, positive values represent right. For y, negative values represent posterior, positive values represent anterior. For z, negative values represent inferior, positive values represent superior.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download