Abstract
Figures and Tables
 | Fig. 1Diagram of the study design.Times of drugs administration, electrocardiogram (ECG) recordings and collections of specimens is shown. Day −2, 2 days before isoproterenol (ISO) administration; day 0, day of first dose of ISO; day +1, day 1 after first dose of ISO; day +2, day 2 after ISO administration; day +5, day 5 after ISO administration. CoPP, cobalt protoporphyrin.
|
 | Fig. 2Electrocardiogram records.From normal control (NC), isoproterenol (ISO), ISO + Trizma, and ISO + cobalt protoporphyrin (CoPP) groups at day 5.
|
 | Fig. 3Effects of cobalt protoporphyrin (CoPP) on myocardial antioxidants.(A) Reduced glutathione (GSH) concentration (mg/g, heart tissue). (B) Superoxide dismutase (SOD) activity (U/g, heart tissue). *Significant vs. normal control (NC) group, #significant vs. isoproterenol (ISO) group, $significant vs. ISO + Trizma group at the same time interval.
|
 | Fig. 4Relative expression of messenger RNA (mRNA) of heme oxygenase-1 (HO-1) in different groups.
*Significant vs. normal control (NC) group, #significant vs. isoproterenol (ISO) group, $significant vs. ISO + Trizma group at the same time interval.
|
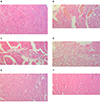 | Fig. 5Heart specimens (H&E staining, 400×).(A) Normal architecture of rat myocardium (normal control group). (B) Infracted zone with interstitial hemorrhage, necrotic changes (isoproterenol [ISO] group). (C) Marked neutrophil infiltration and extensive fibrillary degeneration and interstitial edema (ISO group). (D) Infracted zone with fibrillary degenerations and necrotic changes with extensive necrosis and interstitial edema and marked inflammatory infiltrates (ISO + Trizma group). (E) Mild interstitial edema with mild neutrophil infiltration (ISO + cobalt protoporphyrin [CoPP] group). (F) Normal architecture of myocardium (CoPP group).
|
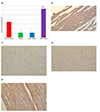 | Fig. 6Expression of connexin 43 (Cx-43).(A) Graph showing the region of interest of Cx-43 expression in the myocardium from different groups. Immunohistochemical stained myocardial specimens (400×) showing a light brown color punctate distribution or rod-shaped pattern in end to end connection (intercalated disc) of Cx-43 (normal control group, B), less light brown color rod-shaped or punctate distribution pattern in end to end connection (intercalated disc) and side to side connection (isoproterenol [ISO] group, C), less light brown color rod-shaped or punctate distribution pattern in end to end connection (ISO + Trizma group, D), and side to side connection more light brown color punctate distribution or rod-shaped pattern in end to end connection (intercalated disc) and side to side connection (ISO + cobalt protoporphyrin [CoPP] group, E). *Significant vs. normal control group, #significant vs. ISO group, $significant vs. ISO + Trizma group at the same time interval.
|
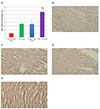 | Fig. 7The expression of Hsp70 in heart tissues.(A) Region of interest ofheat shock protein 70 (Hsp70) expression in myocardium in different groups. Immunohistochemical stained myocardial fibers sections for Hsp70 (400×) showing light brown color in cardiomyocytes cytoplasm (normal control group, B), moderate brown color in cardiomyocytes cytoplasm (isoproterenol [ISO] group, C), moderate brown color in cardiomyocytes cytoplasm (ISO + Trizma group, D), and marked brown color in cardiomyocytes cytoplasm (ISO + cobalt protoporphyrin group, E). *Significant vs. normal control group, #significant vs. ISO group, $significant vs. ISO + Trizma group at the same time interval.
|
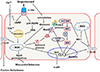 | Fig. 8Graphical abstract showing the proposed mechanisms of isoproterenol (ISO)-induced myocardial infarction (MI) and role of heme oxygenase-1 (HO-1) upregulation.ISO stimulates β1 adrenergic receptors (β1-ARs) which stimulates adenylate cyclase and inhibits phosphodiesterase activity leads to increased cyclic adenosine monophosphate (cAMP) level which result in enhance Ca+2 inflow (via stimulation of L-type Ca+2 channels) results in Ca+2 overload and stimulates p38 mitogen activated protein kinase (MAPK) activity. A raised cytoso1ic Ca+2 may cause myocardial cell death by excessive activation of Ca+2-dependent ATPases and impairing mitochondrial phosphorylation and generation of reactive oxygen species (ROS). Also ISO produces oxidative stress via formation of adrenochromes (quinones) through its autooxidation. ROS causes lipid peroxidation and cell damage and dephosphorylation of gap junctions connexin-43 (Cx-43) leading to cardiac arrhythmias. Also ROS leads to unfolding and aggregation of protein. However, ROS causes nuclear factor erythroid 2-related factor 2 (Nrf2) dissociates from kelch-like ECH-associated protein-1 (Keap-1) and translocates to the nucleus, where binding of Nrf2 to the antioxidant response elements promoter site, leads to the upregulation of HO-1 gene expression. Also cobalt protoporphyrin (CoPP) stimulates upregulation of Nrf2 protein, which induces HO-1 gene expression. HO-1 decreases ROS by its antioxidant action preserving Cx-43 architecture and stimulates HSF1 releasing from the complex with heat shock protein (Hsp), and then it enters the nucleus to bind heat shock elements in the promoter region of HSP70 genes, which leads to their transcription. HSP70 act as molecular chaperons converting aggregates to folded protein.
|
Table 1
Electrocardiogram parameters (heart rate, corrected QT interval and ST segment) in different groups at different time intervals

Values are presented as mean ± standard deviation. CoPP, cobalt protoporphyrin. *Significant vs. normal control (NC) group, #significant vs. isoproterenol (ISO) group, $significant vs. ISO + Trizma group at the same time interval; and asignificant vs. basal value, bsignificant vs. day 2 of the same group.
Table 2
Serum levels of cardiac enzymes (LDH and CK-MB)

Values are presented as mean ± standard deviation. CoPP, cobalt protoporphyrin; LDH, lactate dehydrogenase; CK-MB, creatine kinase-muscle/brain. *Significant vs. normal control (NC) group, #significant vs. isoproterenol (ISO) group, $significant vs. ISO + Trizma group at the same time interval; and asignificant vs. basal value, bsignificant vs. day 2 of the same group.
Table 3
Shows the number of rats and the score of histopathological damage of myocardial tissue in different groups

ACKNOWLEDGEMENTS
Notes
Author contributions S.A.G.E. performed the animal model, experimental procedures and data collection and biochemical assay. A.M.H. performed the statistics, graphs and paper writing. Y.K. and M.H.A. performed the animal model, experimental procedures. A.A.E. and E.F.M. supervised and coordinated the study. G.M.H. performed Real time PCR.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download