Abstract
Figures and Tables
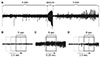 | Fig. 1Data set preparation for deep learning.(A) Non-seizure data were obtained from 5 min of electroencephalogram (EEG) before and after each seizure. (B) Half overlapping 5-sec sliding windows were used to collect non-seizure data. (C) Five-sec segments per 0.25 sec were collected for seizure data to multiply the scarce seizure data. (D) While autodetecting seizure events, the entire EEG was scanned with half overlapping 5-sec sliding windows.
|
 | Fig. 2Examples of convulsive seizures.Seizure activities have different shapes, amplitudes, and durations. Our goal was to determine whether deep learning is adequate to detect different seizure activities.
|
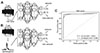 | Fig. 3Classification process for 5-sec electroencephalogram (EEG) segments that were either raw or subjected to spectral analysis.(A) A classifier was built to distinguish total 5,000 raw EEG inputs from 5-sec EEG segments. (B) A classifier was built to distinguish total 100 periodogram results between 0 to 99 Hz. (C) Receiver operating characteristics (ROC) curve of classifiers that learned to distinguish seizure events from either raw data or periodogram results. Area under the curve (AUC) was calculated for each classifier.
|
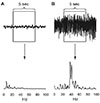 | Fig. 4Examples of the periodogram results for 0 to 99 Hz range.(A) Non-seizure segment. (B) Seizure segment.
|
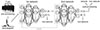 | Fig. 5Classification process for each 5-sec segment.Each 5-sec segment underwent pre-processing to remove large noise and reduce unnecessary computation. The first 100 periodogram values in a 5-sec segment were fed to first deep neural network containing 100 input, 200 and 50 hidden, and 2 output nodes. If it was classified as a seizure, the second network, which contained 100 input, 500 and 125 hidden, and 2 output nodes, classified the same data again. If the second network also classified it as seizure, post-processing finally determined it as seizure if it passed a simple rule-based test. Non-seizure results at any point classified the data as non-seizure and started a new turn with next 5-sec segment.
|
 | Fig. 6Improvement of classification performance by sequential dual deep neural networks.(A) Receiver operating characteristics (ROC) curve and the area under the curve (AUC) when the 5-sec segments of whole training electroencephalogram datasets were classified with the first network only, the second network only, or sequential dual networks. (B) False positive (FP) and false negative (FN) segment numbers were compared after the application of the first network only or sequential dual networks when cut-off threshold for seizures was 0.5 and 0.5 for each network, respectively. (C) FP and FN segment numbers were compared after the application of the first network only or sequential dual networks when cut-off threshold for seizures was 0.99 and 0.90 for each network, respectively.
|
 | Fig. 7Effects of network size and window size on seizure event detection results.(A) Effects of network size. Left panel: false positive (FP) event numbers for training and test datasets for 200, 300, 400, 500, and 600 nodes in the first hidden layer of the second deep neural network. The number of nodes in the second hidden layer was one-fourth that of the first hidden layer. Right panel: false negative (FN) event numbers for training and test datasets for 200, 300, 400, 500, and 600 nodes in the first hidden layer of the second deep neural network. (B) Effects of window size. Left panel: FP event numbers in the training and test datasets for 2-, 5-, and 8-sec windows. Right panel: FN event numbers in the training and test datasets for 2-, 5-, and 8-sec windows.
|
Table 1
Classification profiles of different network structures

True positive (TP), false positive (FP), true negative (TN) and false negative (FN) numbers after different inputs or network structures were applied for deep neural networks. Raw data: classification results of a classifier analyzing raw electroencephalogram inputs. Periodogram 1st: classification results of the first neural network analyzing periodogram. Periodogram dual: classification results of sequential application of the first and the second networks analyzing periodogram. Cut-off: cut-off threshold for classifying the input segment to a seizure. The two cut-off values shown in “Periodogram dual” indicate the cut-off for the first and the second network, respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download