INTRODUCTION
METHODS
Animals
Induction of experimental autoimmune myocarditis
Assessment of myocardial functions
Anti-cardiac myosin antibody and inflammatory cytokines levels
Cell proliferation assay
Histological analysis
Immunohistochemical analysis
Western blotting
Statistical analysis
RESULTS
Effects of andrographolide on mortality, myocardial functions of EAM rats
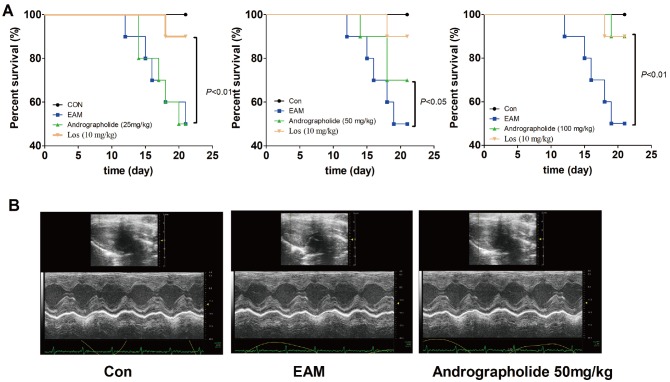 | Fig. 1Effects of andrographolide on mortality of EAM rats.(A) Survival curves of EAM rats. (B) Representative echocardiogram of M-mode on day 21. Control group includes six rats, whereas other groups include ten rats for each. Con: control; EAM: experimental autoimmune myocarditis; Los: losartan.
|
Table 1
Parameters of cardiac function at day 21 after myosin injection
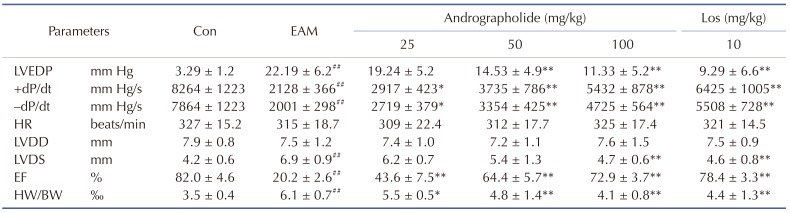
Results are presented as the mean ± S.D. N = 6–9. LVEDP, left ventricular end-diastolic pressure; ±dP/dt, rate of intra-ventricular pressure rise and decline; HR, heart rate; LVDD, left ventricular dimension in diastole; LVDS, left ventricular dimension in systole; EF, ejection fraction; Con: control; EAM: experimental autoimmune myocarditis; Los: losartan. ##p < 0.01, compared with Con; *p < 0.05, **p < 0.01, compared with EAM.
Effects of andrographolide on myocardial histopathology of EAM rats
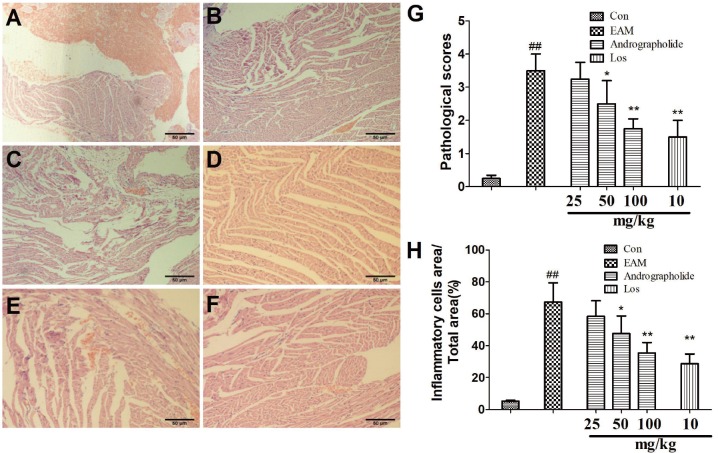 | Fig. 2Effects of andrographolide on myocardial histopathology of EAM rats.(A) Con group; (B) EAM group; (C) andrographolide 25 mg/kg; (D) andrographolide 50 mg/kg; (E) andrographolide 100 mg/kg; (F) losartan 10 mg/kg; (G) pathological scores were evaluated by professional staff. Results are presented as the mean ± S.D. N = 6–9. Con: control; EAM: experimental autoimmune myocarditis; Los: losartan. ##p < 0.01, compared with Con; *p < 0.05, **p < 0.01, compared with EAM.
|
Effects of andrographolide on myocardial inflammatory response in EAM rats
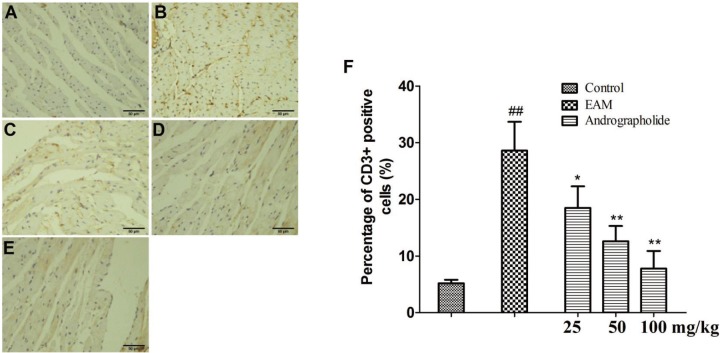 | Fig. 3Effects of andrographolide on the numbers of CD3+ positive cells in myocardium of EAM rats.(A) Con group; (B) EAM group; (C) andrographolide 25 mg/kg; (D) andrographolide 50 mg/kg; (E) andrographolide 100 mg/kg; (F) the numbers of CD3+ positive cells were determined in ten randomly selected fields by a professional staff, who didn't know grouped details. Results are presented as the mean ± S.D. N = 6–9. Con: control; EAM: experimental autoimmune myocarditis; ##p < 0.01, compared with Con; *p < 0.05, **p < 0.01, compared with EAM.
|
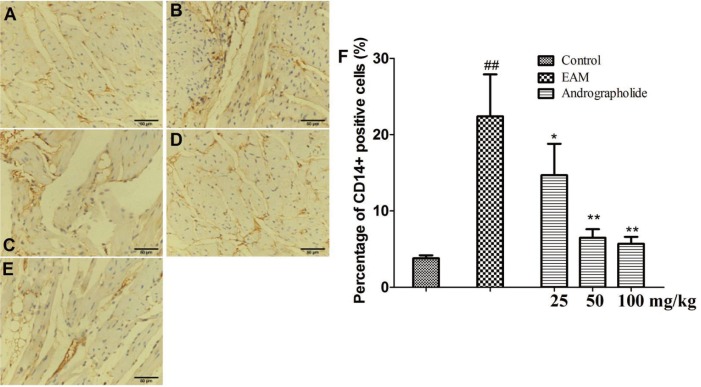 | Fig. 4Effects of andrographolide on the numbers of CD14+ positive cells in myocardium of EAM rats.(A) Con group; (B) EAM group; (C) andrographolide 25 mg/kg; (D) andrographolide 50 mg/kg; (E) andrographolide 100 mg/kg; (F) the numbers of CD14+ positive cells were determined in ten randomly selected fields by a professional staff, who didn't know grouped details. Results are presented as the mean ± S.D. N = 6–9. Con: control; EAM: experimental autoimmune myocarditis; ##p < 0.01, compared with Con; *p < 0.05,**p < 0.01, compared with EAM.
|
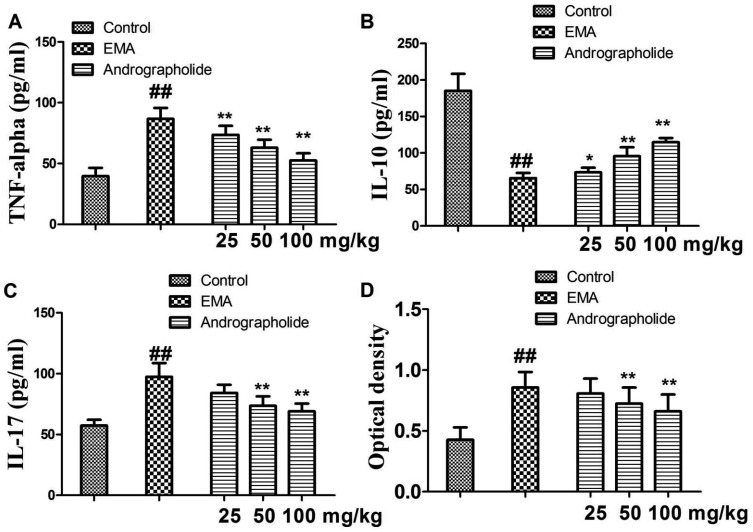 | Fig. 5Effects of andrographolide on the levels of inflammatory cytokines and myosin antibody in EAM rats.(A) TNF-alpha; (B) IL-10; (C) IL-17; (D) myosin antibody. Results are presented as the mean ± S.D. N = 6–9. EAM: experimental autoimmune myocarditis; ##p < 0.01, compared with Control; *p < 0.05, **p < 0.01, compared with EAM.
|
Effects of andro grapholide on PI3K/Akt signaling pathway in EAM rats
 | Fig. 6Effects of andrographolide on PI3K/Akt pathway.(A) Representative stripes; (B, C) the stripes were quantitatively analyzed by Image J. Results are showed as the mean ± S.D. N = 6–9. Con: control; EAM: experimental autoimmune myocarditis. ##p < 0.01, compared with Con; **p < 0.01, compared with EMA.
|
Effects of andrographolide on myosin-induced splenocytes proliferation
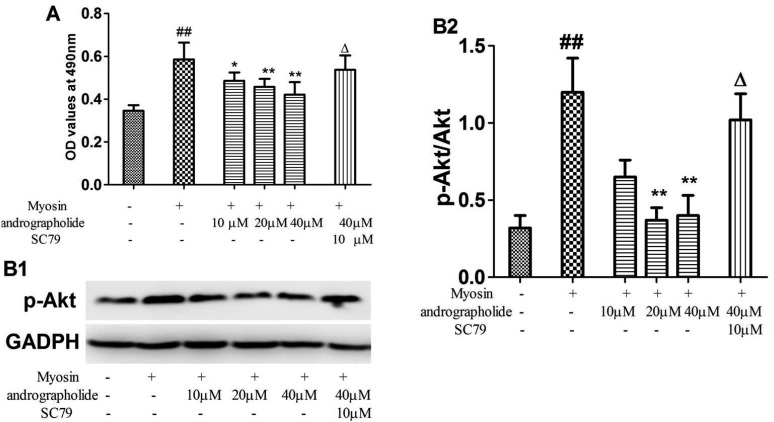 | Fig. 7Effects of andrographolide on proliferation of myosin-treated splenetic cells.(A) Splenetic cells were collected from EAM rats, and treated with andrographolide in the presence and absence of SC79 for 48 h. Myosin-specific proliferative responses were measured by MTT colorimetric technique. (B1) typical p-Akt stripes; (B2) the stripes were quantitatively analyzed by image j. Results are presented as the mean ± S.D. N = 3. Con: control; EAM: experimental autoimmune myocarditis. ##p < 0.01, compared with splenetic cells without treatment; *p < 0.05, **p < 0.01, compared with splenetic cells only treated with myosin (20 µg/ml); Δp < 0.05, compared with the cells treated with myosin and andrographolide (40 µM).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download