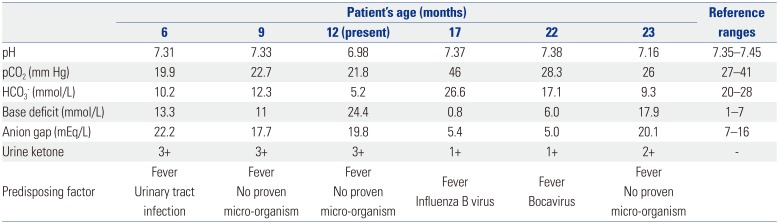
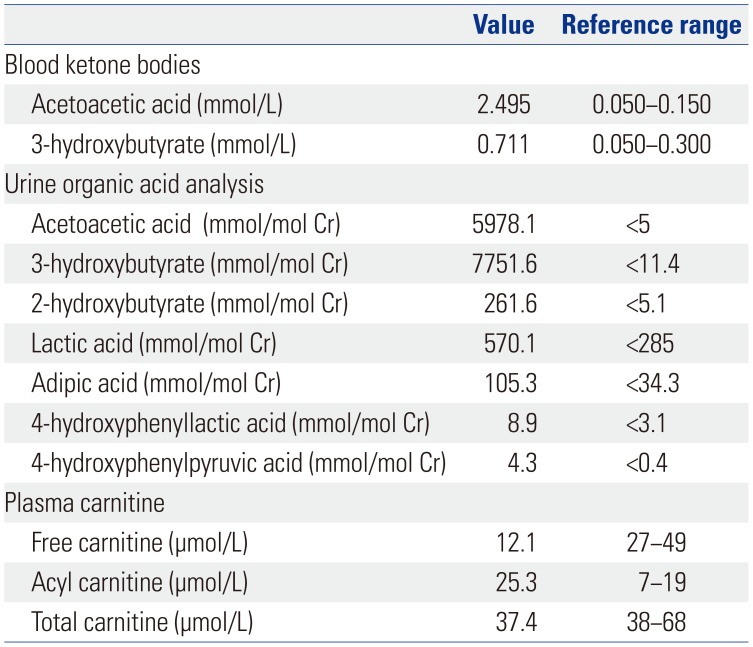
1. Mitchell GA, Fukao T. Inborn errors of ketone body metabolism. In : Scriver C, Beaudet A, Sly W, Valle D, Childs V, Kinzler K, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York (NY): McGraw-Hill, Inc;2001. p. 2327–2356.
2. Tildon JT, Cornblath M. Succinyl-CoA:3-ketoacid CoA-transferase deficiency. A cause for ketoacidosis in infancy. J Clin Invest. 1972; 51:493–498. PMID:
4258782.
3. Berry GT, Fukao T, Mitchell GA, Mazur A, Ciafre M, Gibson J, et al. Neonatal hypoglycaemia in severe succinyl-CoA:3-oxoacid CoA-transferase deficiency. J Inherit Metab Dis. 2001; 24:587–595. PMID:
11757586.

4. Kassovska-Bratinova S, Fukao T, Song XQ, Duncan AM, Chen HS, Robert MF, et al. Succinyl CoA:3-oxoacid CoA transferase (SCOT): human cDNA cloning, human chromosomal mapping to 5p13, and mutation detection in a SCOT-deficient patient. Am J Hum Genet. 1996; 59:519–528. PMID:
8751852.
5. Shafqat N, Kavanagh KL, Sass JO, Christensen E, Fukao T, Lee WH, et al. A structural mapping of mutations causing succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. J Inherit Metab Dis. 2013; 36:983–987. PMID:
23420214.

6. Pretorius CJ, Loy Son GG, Bonnici F, Harley EH. Two siblings with episodic ketoacidosis and decreased activity of succinyl-CoA:3-ketoacid CoA-transferase in cultured fibroblasts. J Inherit Metab Dis. 1996; 19:296–300. PMID:
8803771.

7. Pérez-Cerdá C, Merinero B, Sanz P, Jiménez A, Hernéndez C, Garcáa MJ, et al. A new case of succinyl-CoA: acetoacetate transferase deficiency. J Inherit Metab Dis. 1992; 15:371–373. PMID:
1405472.

8. Hori T, Yamaguchi S, Shinkaku H, Horikawa R, Shigematsu Y, Takayanagi M, et al. Inborn errors of ketone body utilization. Pediatr Int. 2015; 57:41–48. PMID:
25559898.

9. Fukao T, Sass JO, Kursula P, Thimm E, Wendel U, Ficicioglu C, et al. Clinical and molecular characterization of five patients with succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. Biochim Biophys Acta. 2011; 1812:619–624. PMID:
21296660.

10. Longo N, Fukao T, Singh R, Pasquali M, Barrios RG, Kondo N, et al. Succinyl-CoA:3-ketoacid transferase (SCOT) deficiency in a new patient homozygous for an R217X mutation. J Inherit Metab Dis. 2004; 27:691–692. PMID:
15669687.

11. Baric´ I, Sarnavka V, Fumic´ K, Maradin M, Begovic´ D, Ruiter JP, et al. A new case of succinyl-CoA:acetoacetate transferase deficiency: favourable course despite very low residual activity. J Inherit Metab Dis. 2001; 24:81–82. PMID:
11286388.

12. Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014; 37:541–551. PMID:
24706027.

13. Sasai H, Aoyama Y, Otsuka H, Abdelkreem E, Naiki Y, Kubota M, et al. Heterozygous carriers of succinyl-CoA:3-oxoacid CoA transferase deficiency can develop severe ketoacidosis. J Inherit Metab Dis. 2017; 40:845–852. PMID:
28695376.