1. Perkins KM, Boulet SL, Kissin DM, Jamieson DJ. National ART Surveillance (NASS) Group. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol. 2015; 125:70–78. PMID:
25560107.

2. Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006; 107:595–604. PMID:
16507930.

3. Milki AA, Jun SH. Ectopic pregnancy rates with day 3 versus day 5 embryo transfer: a retrospective analysis. BMC Pregnancy Childbirth. 2003; 3:7. PMID:
14604439.

4. Helmy S, Koch M, Kölbl H, Grohmann-Izay B, Solomayer E, Bader Y. Correlation of the volume of ectopic pregnancy and MTX therapy outcome: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2015; 184:108–111. PMID:
25490001.

5. Chang J, Elam-Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, et al. Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ. 2003; 52:1–8.
6. Abou-Setta AM, Mansour RT, Al-Inany HG, Aboulghar MM, Aboulghar MA, Serour GI. Among women undergoing embryo transfer, is the probability of pregnancy and live birth improved with ultrasound guidance over clinical touch alone? A systemic review and meta-analysis of prospective randomized trials. Fertil Steril. 2007; 88:333–341. PMID:
17559845.

7. Teixeira DM, Dassunção LA, Vieira CV, Barbosa MA, Coelho Neto MA, Nastri CO, et al. Ultrasound guidance during embryo transfer: a systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2015; 45:139–148. PMID:
25052773.

8. Zhang B, Cui L, Tang R, Ding L, Yan L, Chen ZJ. Reduced ectopic pregnancy rate on day 5 embryo transfer compared with day 3: a meta-analysis. PLoS One. 2017; 12:e0169837. PMID:
28121989.

9. Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015; 104:899–907. PMID:
26211882.

10. Rombauts L, McMaster R, Motteram C, Fernando S. Risk of ectopic pregnancy is linked to endometrial thickness in a retrospective cohort study of 8120 assisted reproduction technology cycles. Hum Reprod. 2015; 30:2846–2852. PMID:
26428211.

11. Ma NZ, Chen L, Dai W, Bu ZQ, Hu LL, Sun YP. Influence of endometrial thickness on treatment outcomes following in vitro fertilization/intracytoplasmic sperm injection. Reprod Biol Endocrinol. 2017; 15:5. PMID:
28056983.

12. Ku SY, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. A pilot study of the use of low dose human menopausal gonadotropin in ovulation induction. Eur J Obstet Gynecol Reprod Biol. 2003; 109:55–59. PMID:
12818444.

13. Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet. 2010; 282:199–205. PMID:
20182736.

14. Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. Effects of adding luteinizing hormone activity to gonadotropin releasing hormone antagonist protocols may differ according to age. Gynecol Endocrinol. 2010; 26:256–260. PMID:
19757244.

15. Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. Tri-pronucleated zygotes may occur less frequently in luteinizing hormone activity-added cycles. Gynecol Endocrinol. 2011; 27:458–463. PMID:
20642378.

16. Kim YJ, Ku SY, Kim YY, Suh CS, Kim SH, Choi YM. MicroRNA profile of granulosa cells after ovarian stimulation differs according to maturity of retrieved oocytes. Geburtshilfe Frauenheilkd. 2016; 76:704–708. PMID:
27365541.

17. Kim SW, Kim H, Ku SY, Suh CS, Kim SH, Choi YM. A successful live birth with in vitro fertilization and thawed embryo transfer after conservative treatment of recurrent endometrial cancer. Gynecol Endocrinol. 2018; 34:15–19. PMID:
28650773.

18. Kim H, Ku SY, Kim SH, Choi YM, Kim JG. Association between ovarian volume-related dynamic parameters and outcomes of IVF. J Reprod Med. 2017; 62:55–59. PMID:
29999290.
19. Cozzolino M, Vitagliano A, Di Giovanni MV, Laganà AS, Vitale SG, Blaganje M, et al. Ultrasound-guided embryo transfer: summary of the evidence and new perspectives. A systematic review and meta-analysis. Reprod Biomed Online. 2018; 36:524–542. PMID:
29576332.

20. Zakhari A, Ates S, Shaulov T, Dahan MH. Does ovarian reserve affect outcomes in single ideal blastocyst transfers in women less than 40 years of age. Arch Gynecol Obstet. 2018; 297:233–239. PMID:
29082421.
21. Lan VT, Linh NK, Tuong HM, Wong PC, Howles CM. Anti-Müllerian hormone versus antral follicle count for defining the starting dose of FSH. Reprod Biomed Online. 2013; 27:390–399. PMID:
23953069.

22. Alebic MŠ, Stojanovic N, Dewailly D. Discordance between serum anti-Müllerian hormone concentrations and antral follicle counts: not only technical issues. Hum Reprod. 2018; 33:1141–1148. PMID:
29688494.
23. Wu YG, Barad DH, Kushnir VA, Wang Q, Zhang L, Darmon SK, et al. With low ovarian reserve, Highly Individualized Egg Retrieval (HIER) improves IVF results by avoiding premature luteinization. J Ovarian Res. 2018; 11:23. PMID:
29548330.

24. Chalumeau C, Moreau J, Gatimel N, Cohade C, Lesourd F, Parinaud J, et al. Establishment and validation of a score to predict ovarian response to stimulation in IVF. Reprod Biomed Online. 2018; 36:26–31. PMID:
29111311.

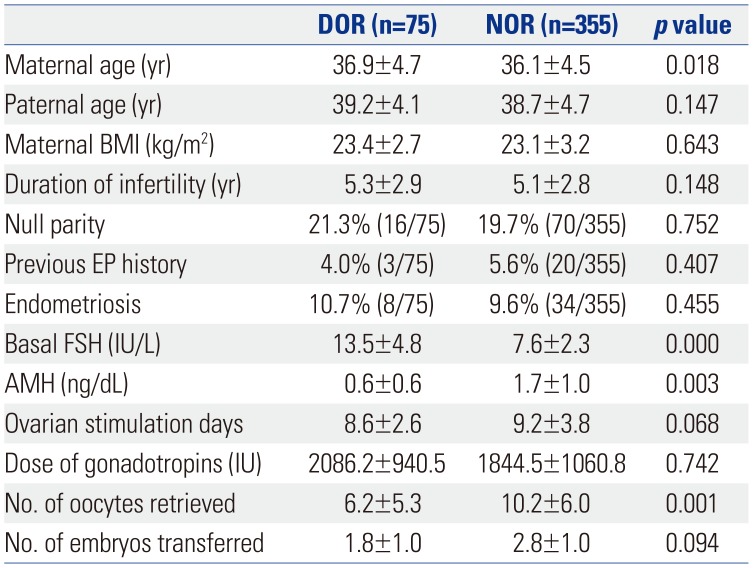
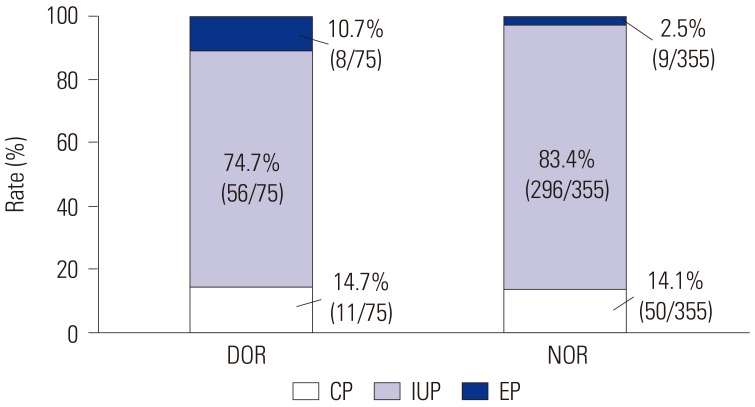
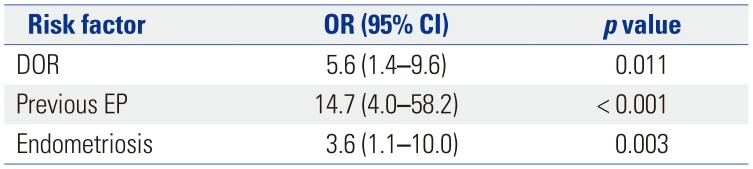
25. Lin S, Yang R, Chi H, Lian Y, Wang J, Huang S, et al. Increased incidence of ectopic pregnancy after in vitro fertilization in women with decreased ovarian reserve. Oncotarget. 2017; 8:14570–14575. PMID:
28099907.

26. Li C, Zhao WH, Zhu Q, Cao SJ, Ping H, Xi X, et al. Risk factors for ectopic pregnancy: a multi-center case-control study. BMC Pregnancy Childbirth. 2015; 15:187. PMID:
26296545.

27. Xiao S, Mo M, Hu X, Zhang H, Xu S, Wang Z, et al. Study on the incidence and influences on heterotopic pregnancy from embryo transfer of fresh cycles and frozen-thawed cycles. J Assist Reprod Genet. 2018; 35:677–681. PMID:
29322346.

28. Xiao X, Zi XD, Niu HR, Xiong XR, Zhong JC, Li J, et al. Effect of addition of FSH, LH and proteasome inhibitor MG132 to in vitro maturation medium on the developmental competence of yak (Bos grunniens) oocytes. Reprod Biol Endocrinol. 2014; 12:30. PMID:
24754924.

29. Weiss A, Beck-Fruchter R, Golan J, Lavee M, Geslevich Y, Shalev E. Ectopic pregnancy risk factors for ART patients undergoing the GnRH antagonist protocol: a retrospective study. Reprod Biol Endocrinol. 2016; 14:12. PMID:
27005813.

30. Malak M, Tawfeeq T, Holzer H, Tulandi T. Risk factors for ectopic pregnancy after in vitro fertilization treatment. J Obstet Gynaecol Can. 2011; 33:617–619. PMID:
21846451.

31. Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, Moon SY, et al. Effect of gonadotropins on human endometrial stromal cell proliferation in vitro. Arch Gynecol Obstet. 2002; 266:223–228. PMID:
12192484.

32. Lee SH, Lee S, Jun HS, Jeong HJ, Cha WT, Cho YS, et al. Expression of the mitochondrial ATPase6 gene and Tfam in Down syndrome. Mol Cells. 2003; 15:181–185. PMID:
12803480.
33. Kim JG, Kim H, Ku SY, Kim SH, Choi YM, Moon SY. Association between human alpha 2-Heremans Schmidt glycoprotein (AHSG) polymorphism and endometriosis in Korean women. Fertil Steril. 2004; 82:1497–1500. PMID:
15589849.
34. Kim SM, Kim SH, Lee JR, Jee BC, Ku SY, Suh CS, et al. Association of leptin receptor polymorphisms Lys109Arg and Gln223Arg with serum leptin profile and bone mineral density in Korean women. Am J Obstet Gynecol. 2008; 198:421. PMID:
18241826.

35. Kim YJ, Kim YY, Kang BC, Kim MS, Ko IK, Liu HC, et al. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J Tissue Eng Regen Med. 2017; 11:3100–3110. PMID:
27717202.

36. Kim YJ, Park KE, Kim YY, Kim H, Ku SY, Suh CS, et al. Effects of estradiol on the paracrine regulator expression of in vitro maturated murine ovarian follicles. Tissue Eng Regen Med. 2017; 14:31–38. PMID:
30603459.

37. Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, Moon SY, et al. Effect of superovulation on the expression of tissue inhibitor of metalloproteinase-3 in the murine endometrium. Gynecol Obstet Invest. 2003; 55:1–6. PMID:
12624544.

38. Yun JW, Kim YY, Ahn JH, Kang BC, Ku SY. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng Regen Med. 2016; 13:323–334. PMID:
30603414.

39. Kim YY, Yun JW, Kim JM, Park CG, Rosenwaks Z, Liu HC, et al. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J Investig Med. 2016; 64:888–893.

40. Kim YJ, Ku SY, Kim YY, Liu HC, Chi SW, Kim SH, et al. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod. 2013; 28:3050–3061. PMID:
23980055.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download