1. Noon L. Prophylactic inoculation against hay fever. Int Arch Allergy Appl Immunol. 1953; 4:285–288. PMID:
13096152.

2. Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003; 33:1076–1082. PMID:
12911781.

3. Blumberga G, Groes L, Haugaard L, Dahl R. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy. 2006; 61:843–848. PMID:
16792582.

4. Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006; 61:855–859. PMID:
16792584.

5. Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012; 129:717–725. PMID:
22285278.
6. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007; 62:943–948. PMID:
17620073.

7. Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004; 114:851–857. PMID:
15480326.

8. Inal A, Altintas DU, Yilmaz M, Karakoc GB, Kendirli SG, Sertdemir Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J Investig Allergol Clin Immunol. 2007; 17:85–91.
9. Reha CM, Ebru A. Specific immunotherapy is effective in the prevention of new sensitivities. Allergol Immunopathol (Madr). 2007; 35:44–51. PMID:
17428399.

10. Hankin CS, Cox L, Lang D, Levin A, Gross G, Eavy G, et al. Allergy immunotherapy among Medicaid-enrolled children with allergic rhinitis: patterns of care, resource use, and costs. J Allergy Clin Immunol. 2008; 121:227–232. PMID:
18206509.

11. Law AW, Reed SD, Sundy JS, Schulman KA. Direct costs of allergic rhinitis in the United States: estimates from the 1996 Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2003; 111:296–300. PMID:
12589348.

12. Anolik R, Schwartz AM, Sajjan S, Allen-Ramey F. Patient initiation and persistence with allergen immunotherapy. Ann Allergy Asthma Immunol. 2014; 113:101–107. PMID:
24814759.

13. Demoly P, Didier A, Mathelier-Fusade P, Drouet M, David M, Bonnelye G, et al. Physician and patient survey of allergic rhinitis in France: perceptions on prevalence, severity of symptoms, care management and specific immunotherapy. Allergy. 2008; 63:1008–1014. PMID:
18691304.

14. Lombardi C, Canonica GW, Passalacqua G. The perception of allergen-specific immunotherapy among chest physicians: an Italian survey. Eur Ann Allergy Clin Immunol. 2014; 46:132–136. PMID:
25053629.
15. Lombardi C, Bettoncelli G, Canonica GW, Passalacqua G. The perception of allergen-specific immunotherapy among Italian general practitioners. Eur Ann Allergy Clin Immunol. 2012; 44:80–82. PMID:
22768727.
16. Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010; (12):CD002893. PMID:
21154351.

17. Bożek A, Kołodziejczyk K, Kozłowska R, Canonica GW. Evidence of the efficacy and safety of house dust mite subcutaneous immunotherapy in elderly allergic rhinitis patients: a randomized, double-blind placebo-controlled trial. Clin Transl Allergy. 2017; 7:43. PMID:
29214012.

18. Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016; 315:1715–1725. PMID:
27115376.
19. Serrano P, Justicia JL, Sánchez C, Cimarra M, Fernández-Távora L, Orovitg A, et al. Systemic tolerability of specific subcutaneous immunotherapy with index-of-reactivity-standardized allergen extracts administered using clustered regimens: a retrospective, observational, multicenter study. Ann Allergy Asthma Immunol. 2009; 102:247–252. PMID:
19354072.

20. Ciprandi G, Cadario G, Valle C, Ridolo E, Verini M, Di Gioacchino M, et al. Sublingual immunotherapy in polysensitized patients: effect on quality of life. J Investig Allergol Clin Immunol. 2010; 20:274–279.
21. Wyrzykowska N, Czarnecka-Operacz M, Adamski Z. Long-term efficacy of allergen specific immunotherapy in atopic dermatitis patients in relation to quality of life. Eur Ann Allergy Clin Immunol. 2015; 47:5–9. PMID:
25599552.
22. Rak S, Yang WH, Pedersen MR, Durham SR. Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: a double-blind, randomised study. Qual Life Res. 2006; 16:191–201. PMID:
17033900.

23. Lombardi C, Senna G, Passalacqua G. Specific immunotherapy among Italian specialists. Allergy. 2006; 61:898–899. PMID:
16792594.

24. Coifman RE, Cox LS. Immunotherapy and Allergy Diagnostics Committee of the AAAAI. 2006 American Academy of Allergy, Asthma & Immunology member immunotherapy practice patterns and concerns. J Allergy Clin Immunol. 2007; 119:1012–1013. PMID:
17418663.
25. Baiardini I, Puggioni F, Menoni S, Boot JD, Diamant Z, Braido F, et al. Patient knowledge, perceptions, expectations and satisfaction on allergen-specific immunotherapy: a survey. Respir Med. 2013; 107:361–367. PMID:
23218454.

26. Antolín-Amerigo D, Tabar IA, Del Mar Fernández-Nieto M, Callejo-Melgosa AM, Muñoz-Bellido FJ, Martínez-Alonso JC, et al. Satisfaction and quality of life of allergic patients following sublingual five-grass pollen tablet immunotherapy in Spain. Drugs Context. 2017; 6:212309. PMID:
29225657.

27. Han DH, Choi YS, Lee JE, Kim DY, Kim JW, Lee CH, et al. Clinical efficacy of sublingual immunotherapy in pediatric patients with allergic rhinitis sensitized to house dust mites: comparison to adult patients. Acta Otolaryngol. 2012; 132(Suppl 1):S88–S93. PMID:
22582789.

28. Frew AJ, Ljørring C, Wolf H, Wüstenberg E, Durham SR, Corrigan CJ, et al. UK Immunotherapy study: reanalysis by a combined symptom and medication score. J Allergy Clin Immunol. 2018; 142:1998–1999. PMID:
30149039.

29. Grouin JM, Vicaut E, Devillier P. Comparison of scores associating symptoms and rescue medication use for evaluating the efficacy of allergy immunotherapy in seasonal allergic rhinoconjunctivitis: results from five trials. Clin Exp Allergy. 2017; 47:254–263. PMID:
27790763.

30. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296. PMID:
23498595.
31. Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, Berin MC. Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol. 2017; 139:189–201. PMID:
27417020.
32. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI). A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013; 5:277–282. PMID:
24003383.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



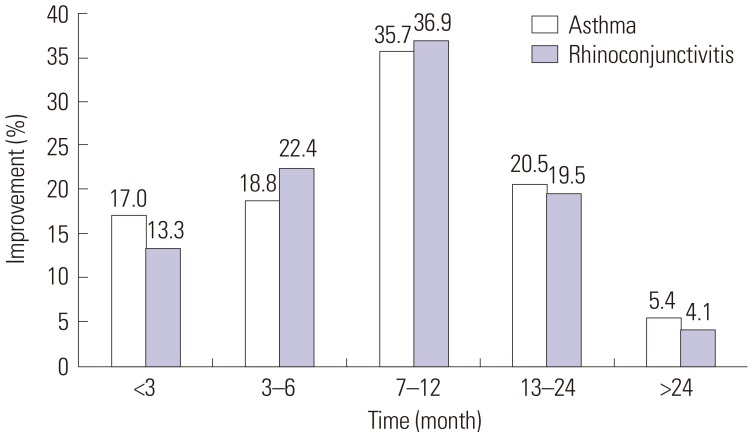
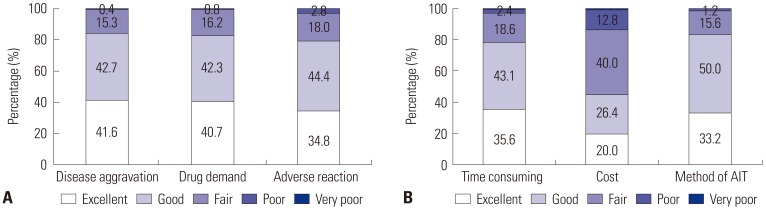
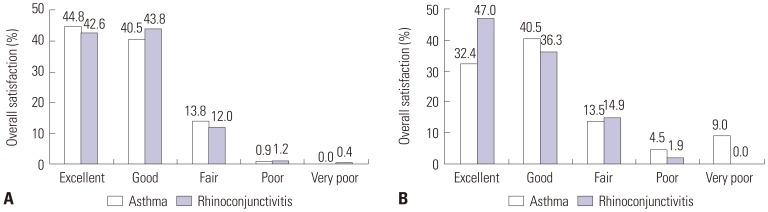
 XML Download
XML Download