1. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014; 383:1490–1502. PMID:
24225001.

2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID:
25651787.

3. Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg. 2009; 394:973–983. PMID:
19582473.

4. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119:1420–1428. PMID:
19487818.

5. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009; 4:199–227. PMID:
18817506.

6. Ju J. Implications of miRNAs in colorectal cancer chemoresistance. Int Drug Discov. 2011; 2011:2063. PMID:
25750759.
7. Ke SB, Qiu H, Chen JM, Shi W, Chen YS. MicroRNA-202-5p functions as a tumor suppressor in colorectal carcinoma by directly targeting SMARCC1. Gene. 2018; 676:329–335. PMID:
30144500.

8. Chen E, Li Q, Wang H, Zhang P, Zhao X, Yang F, et al. MiR-32 promotes tumorigenesis of colorectal cancer by targeting BMP5. Biomed Pharmacother. 2018; 106:1046–1051. PMID:
30119170.

9. Wang D, Wang H, Li Y, Li Q. MiR-362-3p functions as a tumor suppressor through targeting MCM5 in cervical adenocarcinoma. Biosci Rep. 2018; 38:BSR20180668. PMID:
29871972.

10. Yang P, Ni F, Deng RQ, Qiang G, Zhao H, Yang MZ, et al. MiR-362-5p promotes the malignancy of chronic myelocytic leukaemia via down-regulation of GADD45α. Mol Cancer. 2015; 14:190. PMID:
26545365.

11. Xia JT, Chen LZ, Jian WH, Wang KB, Yang YZ, He WL, et al. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J Transl Med. 2014; 12:33. PMID:
24495516.

12. Wang N, Feng Y, Xu J, Zou J, Chen M, He Y, et al. miR-362-3p regulates cell proliferation, migration and invasion of trophoblastic cells under hypoxia through targeting Pax3. Biomed Pharmacother. 2018; 99:462–468. PMID:
29665647.

13. Christensen LL, Tobiasen H, Holm A, Schepeler T, Ostenfeld MS, Thorsen K, et al. MiRNA-362-3p induces cell cycle arrest through targeting of E2F1, USF2 and PTPN1 and is associated with recurrence of colorectal cancer. Int J Cancer. 2013; 133:67–78. PMID:
23280316.

14. Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Adv Cancer Res. 2008; 101:93–126. PMID:
19055944.
15. Kumar JP. The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cell Mol Life Sci. 2009; 66:565–583. PMID:
18989625.

16. Wu W, Ren Z, Liu H, Wang L, Huang R, Chen J, et al. Core promoter analysis of porcine Six1 gene and its regulation of the promoter activity by CpG methylation. Gene. 2013; 529:238–244. PMID:
23954877.

17. Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Six1 increases TGF-beta type I receptor and converts TGF-beta signaling from suppressive to supportive for tumor growth. Cancer Res. 2010; 70:10371–10380. PMID:
21056993.
18. Cheng Q, Ning D, Chen J, Li X, Chen XP, Jiang L. SIX1 and DACH1 influence the proliferation and apoptosis of hepatocellular carcinoma through regulating p53. Cancer Biol Ther. 2018; 19:381–390. PMID:
29333942.

19. Xin X, Li Y, Yang X. SIX1 is overexpressed in endometrial carcinoma and promotes the malignant behavior of cancer cells through ERK and AKT signaling. Oncol Lett. 2016; 12:3435–3440. PMID:
27900017.

20. Lerbs T, Bisht S, Schölch S, Pecqueux M, Kristiansen G, Schneider M, et al. Inhibition of Six1 affects tumour invasion and the expression of cancer stem cell markers in pancreatic cancer. BMC Cancer. 2017; 17:249. PMID:
28388884.

21. Zhang X, Xu R. Six1 expression is associated with a poor prognosis in patients with glioma. Oncol Lett. 2017; 13:1293–1298. PMID:
28454249.

22. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID:
30207593.

23. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–132. PMID:
26808342.

24. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005; 435:834–838. PMID:
15944708.

25. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006; 103:2257–2261. PMID:
16461460.

26. Zou X, Zhong J, Li J, Su Z, Chen Y, Deng W, et al. miR-362-3p targets nemo-like kinase and functions as a tumor suppressor in renal cancer cells. Mol Med Rep. 2016; 13:994–1002. PMID:
26647877.

27. Wu K, Yang L, Chen J, Zhao H, Wang J, Xu S, et al. miR-362-5p inhibits proliferation and migration of neuroblastoma cells by targeting phosphatidylinositol 3-kinase-C2β. FEBS Lett. 2015; 589:1911–1919. PMID:
26073258.

28. Kheirollahi M, Moodi M, Ashouri S, Nikpour P, Kazemi M. Evaluation of miR-362 expression in astrocytoma of human brain tumors. Adv Biomed Res. 2017; 6:129. PMID:
29142892.

29. Ni F, Gui Z, Guo Q, Hu Z, Wang X, Chen D, et al. Downregulation of miR-362-5p inhibits proliferation, migration and invasion of human breast cancer MCF7 cells. Oncol Lett. 2016; 11:1155–1160. PMID:
26893711.

30. Hua L, Fan L, Aichun W, Yongjin Z, Qingqing C, Xiaojian W. Inhibition of Six1 promotes apoptosis, suppresses proliferation, and migration of osteosarcoma cells. Tumour Biol. 2014; 35:1925–1931. PMID:
24114014.

31. Yu C, Zhang B, Li YL, Yu XR. SIX1 reduces the expression of PTEN via activating PI3K/AKT signal to promote cell proliferation and tumorigenesis in osteosarcoma. Biomed Pharmacother. 2018; 105:10–17. PMID:
29807230.

32. Sun SH, Liu D, Deng YT, Zhang XX, Wan DY, Xi BX, et al. SIX1 coordinates with TGFβ signals to induce epithelial-mesenchymal transition in cervical cancer. Oncol Lett. 2016; 12:1271–1278. PMID:
27446426.

33. Jin A, Xu Y, Liu S, Jin T, Li Z, Jin H, et al. Sineoculis homeobox homolog 1 protein overexpression as an independent biomarker for pancreatic ductal adenocarcinoma. Exp Mol Pathol. 2014; 96:54–60. PMID:
24263054.

34. Lv H, Cui A, Sun F, Zhang Y, Li Y, Li L, et al. Sineoculis homeobox homolog 1 protein as an independent biomarker for gastric adenocarcinoma. Exp Mol Pathol. 2014; 97:74–80. PMID:
24866365.

35. Kong J, Zhou X, Liu S, Jin T, Piao Y, Liu C, et al. Overexpression of sineoculis homeobox homolog 1 predicts poor prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014; 7:3018–3027. PMID:
25031720.
36. He Z, Li G, Tang L, Li Y. SIX1 overexpression predicts poor prognosis and induces radioresistance through AKT signaling in esophageal squamous cell carcinoma. Onco Targets Ther. 2017; 10:1071–1079. PMID:
28260921.

37. Xia Y, Zhu Y, Ma T, Pan C, Wang J, He Z, et al. miR-204 functions as a tumor suppressor by regulating SIX1 in NSCLC. FEBS Lett. 2014; 588:3703–3712. PMID:
25157435.

38. O'Brien JH, Hernandez-Lagunas L, Artinger KB, Ford HL. MicroRNA-30a regulates zebrafish myogenesis through targeting the transcription factor Six1. J Cell Sci. 2014; 127(Pt 10):2291–2301. PMID:
24634509.
39. Wang L, Liu H. microRNA-188 is downregulated in oral squamous cell carcinoma and inhibits proliferation and invasion by targeting SIX1. Tumour Biol. 2016; 37:4105–4113. PMID:
26490981.

40. Towers CG, Guarnieri AL, Micalizzi DS, Harrell JC, Gillen AE, Kim J, et al. The Six1 oncoprotein downregulates p53 via concomitant regulation of RPL26 and microRNA-27a-3p. Nat Commun. 2015; 6:10077. PMID:
26687066.

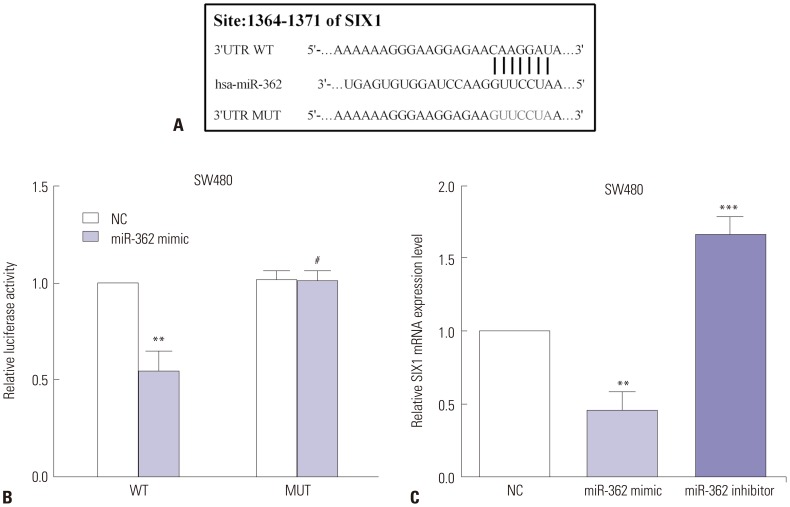
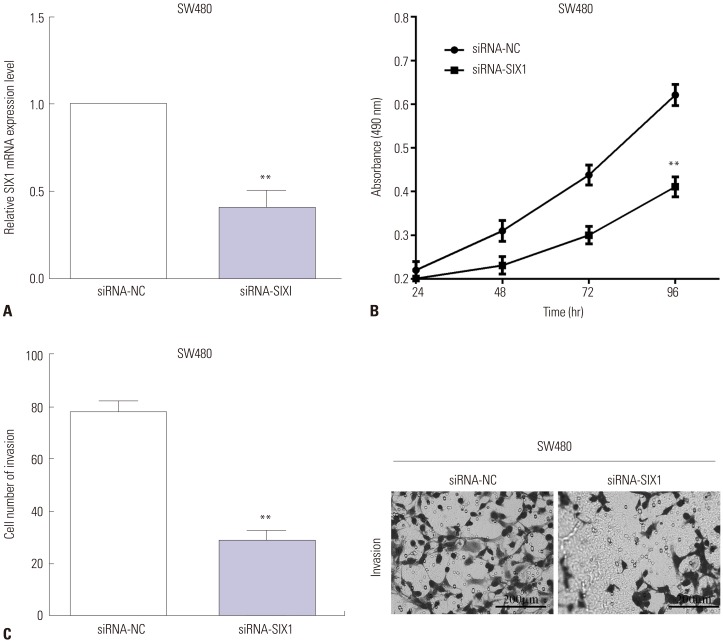
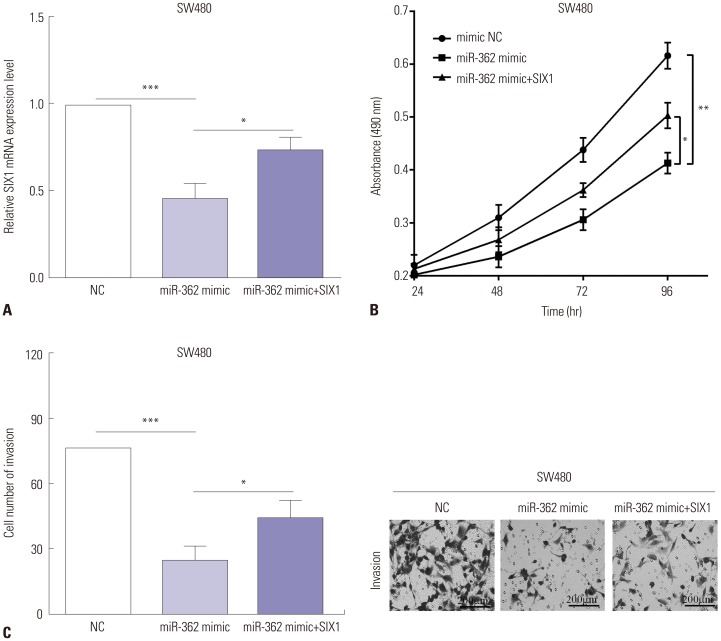




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download