Abstract
ACKNOWLEDGEMENTS
Notes
Author Contribution: Brunner RJ was the principal author who contributed in all facets of the manuscript: initial concept, design, acquisition of literature data, analysis, primary drafter of the article, and manuscript editions. Demeter JH made contributions to the analysis of articles reviewed, writing and editing of the manuscript. Sindhwani P contributed to the concept, design, as well as the analysis of the articles reviewed. He partook in critical revisions and editing of the manuscript.
References
Fig. 1
Flow diagram for the systematic review of leukocytospermia clinical trials. Figure is a flow diagram depicting the search algorithm and results. PubMed, Embase, and Cochrane library was searched for English language articles on human observational studies, clinical trials and randomized control trials using the following terms: “leukocytospermia, pyospermia, and male infertility.” Also included was 4 articles referenced by the various guidelines discussed in the paper. The remaining articles were screened for duplicates and non-english articles were excluded of which there was 4. Another article was deemed ineligible as this study's seminal collection method varied so dramatically from that of the others studied. In total 11 articles are included in this review. N/A: not available.
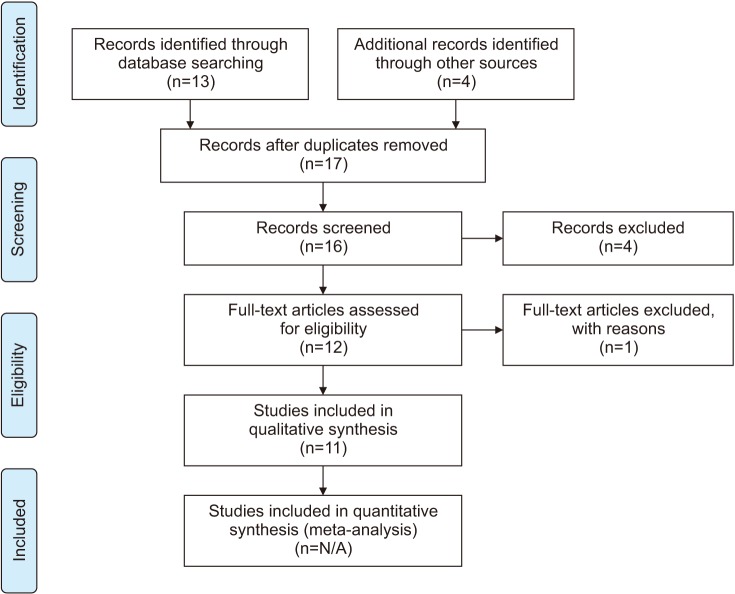
Table 1
Comparison of four major urological and andrological association's guidelines on leukocytospermia
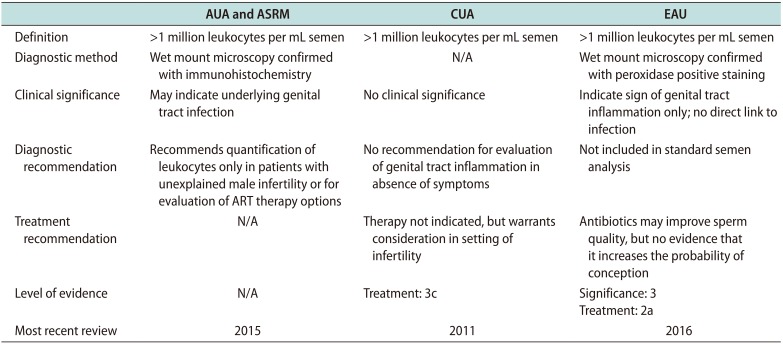
This table reviews the four major urologic and andrologic associations and their diagnostic method, clinical significance, diagnostic and treatment recommendations regarding leukocytospermia. Also reported is the level of evidence and the most recent update to their guidelines. The international bodies that give recommendations and that are included in this table include the American Urologic Association (AUA), the American Society for Reproductive Medicine (ASRM), the Canadian Urological Association (CAU) and the European Association of Urology (EAU). N/A indicates this component was not discussed in the respective guideline article.
Table 2
Comparison of available clinical trials for the treatment of LCS
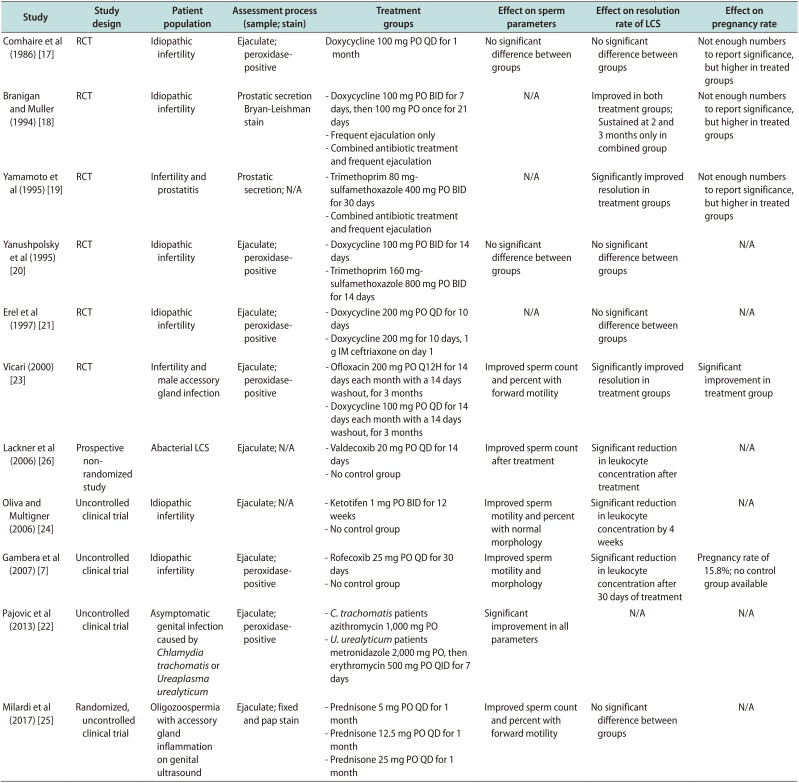
| Study | Study design | Patient population | Assessment process (sample; stain) | Treatment groups | Effect on sperm parameters | Effect on resolution rate of LCS | Effect on pregnancy rate |
|---|---|---|---|---|---|---|---|
| Comhaire et al (1986) [17] | RCT | Idiopathic infertility | Ejaculate; peroxidasepositive | Doxycycline 100 mg PO QD for 1 month | No significant difference between groups | No significant difference between groups | Not enough numbers to report significance, but higher in treated groups |
| Branigan and Muller (1994) [18] | RCT | Idiopathic infertility | Prostatic secretion Bryan-Leishman stain | - Doxycycline 100 mg PO BID for 7 days, then 100 mg PO once for 21 days | N/A | Improved in both treatment groups; Sustained at 2 and 3 months only in combined group | Not enough numbers to report significance, but higher in treated groups |
| - Frequent ejaculation only | |||||||
| - Combined antibiotic treatment and Frequent ejaculation | |||||||
| Yamamoto et al (1995) [19] | RCT | Infertility and prostatitis | Prostatic secretion; N/A | - Trimethoprim 80 mgsulfamethoxazole 400 mg PO BID for 30 days | N/A | Significantly improved resolution in treatment groups | Not enough numbers to report significance, but higher in treated groups |
| - Combined antibiotic treatment and frequent ejaculation | |||||||
| Yanushpolsky et al (1995) [20] | RCT | Idiopathic infertility | Ejaculate; peroxidasepositive | - Doxycycline 100 mg PO BID for 14 days | No significant difference between groups | No significant difference between groups | N/A |
| - Trimethoprim 160 mgsulfamethoxazole 800 mg PO BID for 14 days | |||||||
| Erel et al (1997) [21] | RCT | Idiopathic infertility | Ejaculate; peroxidasepositive | - Doxycycline 200 mg PO QD for 10 days | N/A | No significant difference between groups | N/A |
| - Doxycycline 200 mg for 10 days, 1 g IM ceftriaxone on day 1 | |||||||
| Vicari (2000) [23] | RCT | Infertility and male accessory gland infection | Ejaculate; peroxidasepositive | - Ofloxacin 200 mg PO Q12H for 14 days each month with a 14 days washout, for 3 monthsx | Improved sperm count and percent with forward motility | Significantly improved resolution in treatment groups | Significant improvement in treatment group |
| - Doxycycline 100 mg PO QD for 14 days each month with a 14 days washout, for 3 months | |||||||
| Lackner et al (2006) [26] | Prospective nonrandomized study | Abacterial LCS | Ejaculate; N/A | - Valdecoxib 20 mg PO QD for 14 days | Improved sperm count after treatment | Significant reduction in leukocyte concentration after treatment | N/A |
| - No control group | |||||||
| Oliva and Multigner (2006) [24] | Uncontrolled clinical trial | Idiopathic infertility | Ejaculate; N/A | - Ketotifen 1 mg PO BID for 12 weeks | Improved sperm motility and percent with normal morphology | Significant reduction in leukocyte concentration by 4 weeks | N/A |
| - No control group | |||||||
| Gambera et al (2007) [7] | Uncontrolled clinical trial | Idiopathic infertility | Ejaculate; peroxidase-positive | - Rofecoxib 25 mg PO QD for 30 days | Improved sperm motility and morphology | Significant reduction in leukocyte concentration after 30 days of treatment | Pregnancy rate of 15.8%; no control group available |
| - No control group | |||||||
| Pajovic et al (2013) [22] | Uncontrolled clinical trial | Asymptomatic genital infection caused by Chlamydia trachomatis or Ureaplasma urealyticum | Ejaculate; peroxidase-positive | - C. trachomatis patients azithromycin 1,000 mg PO | Significant improvement in all parameters | N/A | N/A |
| - U. urealyticum patients metronidazole 2,000 mg PO, then erythromycin 500 mg PO QID for 7 days | |||||||
| Milardi et al (2017) [25] | Randomized, uncontrolled clinical trial | Oligozoospermia with accessory gland inflammation on genital ultrasound | Ejaculate; fixed and pap stain | - Prednisone 5 mg PO QD for 1 month | Improved sperm count and percent with forward motility | No significant difference between groups | N/A |
| - Prednisone 12.5 mg PO QD for 1 month | |||||||
| - Prednisone 25 mg PO QD for 1 month |
This table summaries English language studies found in our literature search or referenced within the various guidelines. This includes 11 observational or clinical trials for the treatment of LCS. Treatment methods are reported in the table including antibiotics, frequent ejaculation, anti-histamines, and corticosteroids. Described for each study is the author, the study design, patient population, assessment process, treatment groups, effect on sperm parameters, effect on resolution rate of LCS and the effect on pregnancy rate. N/A indicates that this component was not indicated or studied.
LCS: leukocytospermia, RCT: randomized control trials, PO: per os, QD: every day, BID: twice a day, IM: intramuscular, QID: four times a day.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download