Abstract
Wilson's disease shows considerably various symptoms that sometimes timely diagnosis is delayed when patient shows vague clinical presentation. We present a female patient whose initial symptom was hypersomnia and severe fatigue. She was initially diagnosed as depression. Because initial hepatic function test was unremarkable, it was not easy to come to think about relationship between hepatic function and hypersomnia. Her final diagnosis was Wilson's disease. This case suggested that hypersomnia otherwise unexplained could be the presenting symptom of Wilson's disease.
Wilson's disease (WD), also known as hepatolenticular degeneration, is an autosomal recessive genetic disorder of copper metabolism leading to hepatic dysfunction and various neurological or psychiatric symptoms by copper accumulation in tissue. It is due to mutations in the WD gene (ATP7B) which has been mapped to choromosome 13 (13q14.3) and is expressed primarily in the liver, kidney, and placenta. The main targets of copper accumulation are the liver and the brain, so hepatic dysfunction and neuropsychiatric symptoms are the core features that can lead to diagnosis (1). People with hepatic dysfunction tend to come to diagnosis earlier as children than those with psychiatric and neurological symptoms, who tend to be older. The neuropsychiatric symptoms include parkinsonism, seizure, cognitive dysfunction, depression, anxiety and psychosis(2). Because the clinical spectrum of symptomatic presentation of WD is very wide, it is important to know the various clinical presentations.
A 46-year-old woman visited our clinic because of excessive amount of sleeping which started 2 years ago. Before the onset of symptom, she seldom felt fatigue even after 20 kilometer-walk a day. However, at the onset of an episode, she became very drowsy and progressively spent most of the day and night for sleeping without physically laborious work. She usually slept for more than 16 hours a day and sometimes for 5 days continuously, waking only to go to the bathroom or eating. When she was awake, she appeared absent-minded and exhausted. At first, she was assessed in a psychiatry clinic. Twenty four-hour polysomnographic monitoring was performed and it revealed increased respiratory disturbance index and severe snoring but did not meet the criteria of narcolepsy (Table 1). Sometimes she was emotionally labile that shed tears easily and showed irritability to her husband that made frequent quarrel between them. So, she was treated as major depressive disorder combined with obstructive sleep apnea for 2 years. But she did not show improvement on various anti-depressants. She also had work up for ENT surgery for OSA but didn't meet the criteria of operation or continuous positive airway pressure (CPAP) therapy. However, the symptoms progressed from day to day up to the degree that she started to have gait disturbance with frequent falling and remarkably decreased concentration. At this state, she was admitted referred to our hospital.
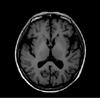
At initial work up, the Korean version of the mini-mental state examination (K-MMSE) revealed a score of 29/30, reflecting normal but detailed neuropsychological tests showed multiple cognitive deficits, including attention, visuospatial function and memory. Neurological examination revealed slow mentation, bilateral positive Babinski sign with generalized hyperreflexia and dysesthesia on both lower extremities. She had a history of well controlled DM and hypothyroidism. Routine laboratory finding revealed moderately increased aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase and total bilirubin (198 IU/L, 117 IU/L, 189 IU/L and 1.7 mg/dL, respectively). Anti-HAV IgM, HBsAg, anti-HCV, EBV, CMV marker and autoantibodies (FANA, mitochondrial antibody, smooth muscle antibody) showed negative result. Abdominal ultrasonography showed diffuse fatty liver. She had no history of alcohol consumption, herbal medicines. Liver CT revealed large amount of ascites, fatty liver, hepatomegaly which was compatible with acute hepatic dysfunction (Fig. 1). Detection of abnormal liver function without definite etiologic finding led to extensive investigation. Low serum copper (25 µg/dL; normal range 70-130 µg/dL), low serum ceruloplasmin (12.6 mg/dL; normal range 15-40 mg/dL) and high urinary copper excretion (931 µg/dL/24 h; normal range 38-70 µg/dL/24 h) were found. Kayser-Fleischer corneal rings were not observed. Liver biopsy showed steatosis with diffuse hepatocyte ballooning, Mallory bodies, inflammatory cell and high copper content (314 µg/g; normal range 10-35 µg/g weight). Lumbar puncture revealed normal cerebrospinal fluid profile. EEG was normal. The brain MRI showed subtle change of bilateral globus pallidus in T1-weighed image which might be related to poor liver function (Fig. 2). On the basis of the findings of high level urinary copper excretion, low serum copper level and high copper content in liver, diagnosis of WD was established. She has no family history. Molecular genetic study of the ATP7B gene in the patient were done with informed consent. DNA sequencing of exons from 1 to 21 showed no known mutation, novel variant, deletion or duplication.
Treatment with D-penicillamine was started and her symptoms including hypersomnia improved gradually. But one year after initiation of treatment, she started to complain fatigue again but sleepiness was not so severe compared with the state before the treatment. So, methylphenidate 20mg per day was added and it relieved her symptom remarkably. At present, her fatigue still wax and wane according to the level of AST and ALT. She is on maintenance dose of 500mg of D-penicillamine with zinc but liver function have been deteriorated progressively that she is waiting for liver transplantation.
According to previous study, the patients with WD were prone to have sleep disturbance (3). Compared with the control group, patients with WD have higher rate of excessive daytime sleepiness, cataplexy-like episode, daytime napping with tiredness and poor nocturnal sleep. They also have lower latency of stage 1 and stage 2 of non-rapid eye movement (NREM) sleep and less amount of NREM sleep stage 2. Also one third of the patients showed short or borderline multiple sleep latency test (MSLT) values. But, our patient showed increased amount of NREM sleep stage 2 (62.8%) and decreased amount of NREM sleep stage 3 and 4 (0%) which meant disrupted conversion to deeper stage of sleep. She also had obstructive sleep apnea (OSA) of mild degree which did not need operation or CPAP according to the opinion of ENT specialist. So, mild OSA could not explain severe fatigue and somnolence which persisted all day long.
It was not easy to hypothesize that altered liver function might be the cause of hypersomnolnece. On top of it, her liver function test was not remarkable when she first visited psychiatric clinic due to severe fatigue and hypersomnia (AST: 46 IU/L, ALT: 16 IU/L). So, she got delayed diagnosis and treated as depression with OSA for more than 2 years. Although WD revealed a wide spectrum of clinical symptoms and showed high frequency of sleep co-morbidity, hypersomnia may be underdiagnosed when it comes to first main symptom. This can be found in previously reported case of a patient with WD with hypersomnia confirmed by 24-h cassette EEG polysomnographic monitoring (4). In that case, patient became free of symptom on penicillamine treatment. Althoug hypersomnia of our patient responded to penicillamine, but fatigue with mild sleepiness relapsed in a year that methylphenidate was added. These differences between two cases might be due to the degree of hepatic dysfunction. In case of our patient, it seems that she had worse hepatic function because of delayed diagnosis than previously reported case.
The pathophysiologic mechanism of hypersomnia in WD is unknown. Among chronic liver disease, primary biliary cirrhosis (PBC) leading to chronic liver dysfunction shows excessive daytime sleepiness and profound fatigue (5). It was reported that patient with PBC had lower sleep quality with remarkably increased amount of time spent during the day compared with normal controls (6). Forton and collegues reported that manganese deposition may occur in the globus pallidus (GP) in PBC and it might be the important mechanism in the genesis of fatigue in patients with PBC. This high signal change within GP in T1-weighed brain MRI image was also observed in our patient so the hypersomnia and fatigue of our patient might be related to hepatic dysfunction induced manganese deposition in the GP. Because the symptom of our patient is very similar to that of patient with PBC which is described above, it would be possible that they have something in common in view of their mechanism that still remains unclear. Some interesting finding is that our patient complained relapsed fatigue with mild somnolence as hepatic function deteriorated in a year after initiation of D-penicillamine. But the characteristic symptom of hypersomnia was not observed after treatment of D-penicillamine even when she showed significantly elevated liver markers although she complained fatigue. So the hypersomnia which was observed before might be predominantly related to brain dysfunction rather than direct effect of liver dysfunction by WD. It is supported by the fact that her initial liver marker was not remarkable while she was treated for hypersomnia in psychiatric clinic.
The genetic defect of WD is linked to the ATP7B gene on chromosome13 (7). The sensitivity of molecular genetic testing for WD was initially reported as 80% (8), and subsequent studies using more sensitive DNA sequencing methods have raised the sensitivity to greater than 95% (http://www.ncbi.nlm.nih.gov/books/NBK1512/). But in our patient and previously reported patient of WD with hypersomnia didn't show any mutation.
Because phenotypes of WD are considerably variable, diagnosis in time remains a challenge so at least half of patients with WD are never diagnosed (9). If timely diagnosis and treatment is absent, hepatic and neurologic dysfunction would progress very rapidly. So, early and accurate diagnosis is critical. In this case report we confirmed hypersomnia and severe fatigue as an initial symptom of WD and we put an emphasis on early diagnosis of WD although it has various spectrum of phenotypes.
Figures and Tables
Fig. 1
Liver CT of the patient. Large amount of ascites and moderate to severe degree of fatty liver with hepatomegaly was observed.

Fig. 2
Brain MRI of the patient. Subtle T1-weighed high signal intensity at bilateral globus pallidus.
References
3. Nevsimalova S, Buskova J, Bruha R, Kemlink D, Sonka K, Vitek L, et al. Sleep disorders in Wilson's disease. Eur J Neurol. 2011; 18:184–190.

4. Firneisz G, Szalay F, Halasz P, Komoly S. Hypersomnia in Wilson's disease: an unusual symptom in an unusual case. Acta Neurol Scand. 2000; 101:286–288.

5. Newton JL, Gibson GJ, Tomlinson M, Wilton K, Jones D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology. 2006; 44:91–98.

6. Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997; 82:1313–1316.

7. Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008; 47:2089–2111.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download