An 8-year-old boy who presented with an acute condition and difficulty of breathing visited the emergency department of our institution. He had been diagnosed with autism and was attending a special-education school. Although the patient had been diagnosed with asthma, he had never undergone appropriate treatment for it. The patient had had a fever without any evident respiratory symptoms within 1 week prior to the visit. He had been diagnosed with acute pharyngotonsillitis and treated with antibiotics of unknown type. Although he did not have fever afterward, he tended to fall asleep and seemed to lack energy at school and had breathing difficulty; hence, he visited our hospital. At the time of visit, a stridor sound when breathing, cough, and phlegm were noted. During the physical examination, tachypnea (respiratory rate >36/min) and lethargy were observed. Wheezing and rales were heard in both lungs upon auscultation. Percutaneous oxygen saturation (SpO
2) was only 88% when measured while administering oxygen at a high concentration of 10 L/min. The patient did not respond to short β2-agonist bronchodilator (Ventolin
®; GlaxoSmithKline, Brentford, UK) and glucocorticoid inhalation therapy. Initial chest X-ray scans showed diffusely increased opacity and moderate pleural effusion in both lungs (
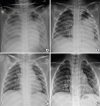
Fig. 1).
The results of the initial venous blood gas analysis performed at the time of visit were as follows: pH, 7.27; partial pressure of CO
2, 44 mmHg; partial pressure of oxygen, 70 mmHg; bicarbonate, 20.2 mEq/L; base excess, −6.5; SpO
2, 91% (100% oxygen administered at 10 L/min); white blood cell (WBC) count, 17,100/µL (segment neutrophil 82.1%) indicative of leukocytosis; and C-reactive protein, 15.0 mg/dL. The serum aspartate aminotransferase (AST) level was 6,536 U/L; alanine aminotransferase (ALT), 1,083 U/L; blood urea nitrogen (BUN), 22.0 mg/dL; and creatinine (Cr), 0.96 mg/dL (
Table 1). Under the suspicion of pneumonia caused by bacteria or MP infection, cefotaxime and clarithromycin were intravenously administered, and ventilator care was started. However, even with the ventilator setting with the pressure control (assist control mode), positive end expiratory pressure (PEEP) of 12 mmHg, peak inspiratory pressure (PIP) of 20 mmHg, fraction of inspired oxygen (FiO
2) of 100%, and respiratory rate (RR) of 24/min, the oxygen index was 22.0 and oxygen saturation index was 16.5, which indicates severe ARDS. The pulse oximeter showed SpO
2 of ≤92%, and lung parenchymal infiltration worsened. On hospital day 2, echocardiography was performed due to ARDS and multiple organ failure affecting the liver and kidneys. After confirming that the patient had a normal cardiac function, veno-venous (right common femoral vein drainage and right internal jugular vein infusion) ECMO was administered. A mycoplasma serology test performed upon admission showed mycoplasma immunoglobulin (Ig) M (+, 5.1; positive ≥1.1 index, ELISA; DIESSE, Siena, Italy), IgG (+, 100 AU/mL, positive ≥18 AU/mL, ELISA; DIESSE), and cold agglutinin 1:256. In the MP polymerase chain reaction (PCR) test (Seeplex
® PneumoBacter ACE Detection (V3.0); Seegene Inc., Seoul, Korea) from transtracheal aspirates, the patient tested positive for MP (
Table 2). The lactate dehydrogenase (LDH) level was elevated above the maximum normal limit (11,000 U/L) (
Table 1). On hospital day 2, serum concentration levels of IgG, IgA, IgM, and IgE were 338 mg/dL (reference range, 608–1,572 mg/dL), 38 mg/dL (reference range, 43–207 mg/dL), 46 mg/dL (reference range, 33–236 mg/dL) and 11.1 IU/mL (reference range, 0–230 IU/dL). In addition, 400 mg/kg/day of intravenous IgG was administered for 5 days (total 2 g/kg/day). Chest tube insertion was performed due to pleural effusion that persisted in both lungs for 2 hospital days. In a pleural fluid test, a serosanguinous colored fluid was drained from the both lungs (pleural fluid analysis results: red blood cell 70,000/μL, WBC 190/μL, polymorphonuclear neutrophil 80%, pH 7.6, glucose 99 mg/dL, and protein 3.3 g/dL). A culture of the pleural fluid did not grow any organisms. After the use of ECMO, azotemia, Cr, LDH, and blood cytokine levels started to increase on hospital day 2, and CRRT was performed. ECMO was used for a total of 17 days and was weaned on hospital day 18 without any complications (
Fig. 2). CRRT was weaned on hospital day 34 after monitoring the urine output. The maximum serum Cr level was reached on hospital day 7 (4.29 mg/dL). AST level increased up to 10,258 U/L on hospital day 3, and ALT level increased up to 1,447 U/L on hospital day 2. On hospital day 11, improvements on simple chest radiographs were noted, and the pleural effusion also appeared to improve. The chest tubes were removed from the left lung on hospital day 17 and from the right lung on hospital day 22. ECMO was removed after observing stable blood gas levels on hospital day 18. The patient was continuously treated with a mechanical ventilator with the following settings: pressure control (assist control) mode, PEEP 10 mmHg, PIP 18 mmHg, RR 20/min, and FiO
2 55%.
Due to poor response to antibiotic treatment, cefotaxime was changed to meropenem, and multidrug-resistant Acinetobacter baumannii was noted from tracheal aspirate cultures on hospital day 11. Endotracheal extubation was consistently attempted, but antibiotics had to be continuously used due to fever. Antibiotics were used due to repeated atelectasis and hospital-acquired infection (multidrug-resistant A. baumannii was isolated in the transtracheal aspiration on hospital day 12 and Candida albicans was isolated in the transtracheal aspiration and urine culture on hospital day 31), and weaning failure persisted. For effective lung care, tracheostomy was performed on hospital day 43, and the patient was transferred to a general ward on hospital day 52 to continue the treatment. The patient underwent pulmonary rehabilitative therapy, and the tracheal tube was removed on hospital day 101. In the last blood test performed before discharge, the patients blood component levels were found to have returned to normal with BUN of 14.7 mg/dL and serum Cr of 0.27 mg/dL. The serum AST and ALT levels were also decreased to 39 and 65 IU/mL, respectively. Although kidney sonography revealed mildly increased echogenicity of its cortex on hospital day 15, no evidence of kidney injury was found in a test performed before discharge. Brain magnetic resonance imaging (MRI) performed on hospital day 111 did not show any evidence of ischemic injury, infarction, or hemorrhage. The patient was discharged without respiratory support on hospital day 113. The patient was continuously treated with Ventolin® (GlaxoSmithKline), budesonide, and inhalation therapy after discharge.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download