Abstract
Purpose
We aimed to investigate the epidemiological characteristics of Staphylococcus aureus bacteremia in Korean children.
Methods
We retrospectively collected and analyzed data from the medical records of the patients with S. aureus bacteremia ≤18 years of age in Gil Medical Center from 2002 to 2016.
Results
A total of 212 SAB cases were detected. The annual incidence of SAB from 2002 to 2016 ranged from 0.77 to 1.95 per 1,000 patients hospitalized. The neonate group (<28 days of age) and the pediatric group (28–18 years of age) were 51.4% (n=109) and 48.6% (n=103), respectively. According to the origin of infection, there were 93 cases (43.9%) of community-associated (CA)-SAB and 119 cases (56.1%) of healthcare-associated (HA)-SAB. The rates of HA-SAB among the neonate group and among the pediatric group were 64.2% and 47.6%, respectively (P=0.015). There was no difference in complications between CA-SAB and HA-SAB, but mortality was higher in HA-SAB. The proportion of methicillin-resistance S. aureus (MRSA) was the highest in neonates (88.1%), decreased with age, and was 36.4%–37.5% among children aged ≥5 years. The MRSA proportion was 72.2%, showing no consistent trend over the period.
Go to : 
References
1. Kaplan SL, Hulten KG, Mason EO. Staphylococcus aureus infections (Coagulase-positive Staphylococci). Cherry JD, Harrison GJ, Kaplan SL, Hotez PJ, editors. editors.Feigin and Cherry's textbook of pediatric infectious diseases. 7th ed.Philadelphia, PA: Elsevier Saunders;2014. p. 1113–30.
2. Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002). Diagn Microbiol Infect Dis. 2004; 50:59–69.

3. Rhie K, Choi EH, Cho EY, Lee J, Kang JH, Kim DS, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (2006–2010): a retrospective multicenter study. J Korean Med Sci. 2018; 33:e45.

4. Lee JH, Cho HK, Kim KH, Kim CH, Kim DS, Kim KN, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (1996–2005): a retrospective multicenter study. J Korean Med Sci. 2011; 26:174–83.

5. Le J, Dam Q, Tran T, Nguyen A, Adler-Shohet FC, Kim S, et al. Epidemiology and hospital readmission associated with complications of Staphylococcus aureus bacteremia in pediatrics over a 25-year period. Epidemiol Infect. 2017; 145:2631–9.
6. Frederiksen MS, Espersen F, Frimodt-M⊘ller N, Jensen AG, Larsen AR, Pallesen LV, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J. 2007; 26:398–405.
7. Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017; 21:211.

8. Cobos-Carrascosa E, Soler-Palacín P, Nieves Larrosa M, Bartolomé R, Martín-Nalda A, Antoinette Frick M, et al. Staphylococcus aureus bacteremia in children: changes during eighteen years. Pediatr Infect Dis J. 2015; 34:1329–34.
9. Park J, Kim S, Lee J. Infection of methicillin-resistant S. aureus nasal carriage in the community pediatric population. Korean J Epidemiol. 2006; 28:171–81.
10. Choe YJ, Lee SY, Sung JY, Yang MA, Lee JH, Oh CE, et al. A review of Staphylococcus aureus infections in children with an emphasis on community-associated methicillin-resistant S. aureus infections. Korean J Pediatr Infect Dis. 2009; 16:150–61. CROSSREF.
11. Suryati BA, Watson M. Staphylococcus aureus bacteraemia in children: a 5-year retrospective review. J Paediatr Child Health. 2002; 38:290–4.
12. Denniston S, Riordan FA. Staphylococcus aureus bacteraemia in children and neonates: a 10 year retrospective review. J Infect. 2006; 53:387–93.
13. Ross AC, Toltzis P, O'riordan MA, Millstein L, Sands T, Redpath A, et al. Frequency and risk factors for deep focus of infection in children with Staphylococcus aureus bacteremia. Pediatr Infect Dis J. 2008; 27:396–9.
14. Hill PC, Wong CG, Voss LM, Taylor SL, Pottumarthy S, Drinkovic D, et al. Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand. Pediatr Infect Dis J. 2001; 20:868–73.
15. McMullan BJ, Bowen A, Blyth CC, Van Hal S, Korman TM, Buttery J, et al. Epidemiology and mortality of Staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatr. 2016; 170:979–86.
16. Asgeirsson H, Gudlaugsson O, Kristinsson KG, Vilbergsson GR, Heiddal S, Haraldsson A, et al. Low mortality of Staphylococcus aureus bacteremia in Icelandic children: nationwide study on incidence and outcome. Pediatr Infect Dis J. 2015; 34:140–4.
17. Lee YJ, Byun SY, Park SE, Park HJ. Risk factors associated with complicated methicillin-resistant Staphylococcus aureus bacteremia in neonates. Korean J Pediatr. 2010; 53:173–7. CROSSREF.
18. Na DC, Lee JH, Lee WU, Kim ER. Carriage Rates of Methicillin-resistant Staphylococcus aureus in neonates with neonatal jaundice. Korean J Pediatr Infect Dis. 2011; 18:143–53. CROSSREF.
19. Hakim H, Mylotte JM, Faden H. Morbidity and mortality of Staphylococcal bacteremia in children. Am J Infect Control. 2007; 35:102–5.

20. Ayliffe GA. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997; 24(Suppl 1):S74–9.
22. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002; 110:285–91.

23. Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006; 5:2.

24. Song JS, Choe PG, Song KH, Cho JH, Kim SH, Bang JH, et al. Multicenter study for frequency and clinical features of community-associated methicillin-resistant Staphylococcus aureus in Korea. Infect Chemother. 2006; 38:325–33.
25. Pérez G, Martiren S, Reijtman V, Romero R, Mastroianni A, Casimir L, et al. Community-acquired Staphylococcus aureus bacteremia in children: a cohort study for 2010–2014. Arch Argent Pediatr. 2016; 114:508–13.
Go to : 
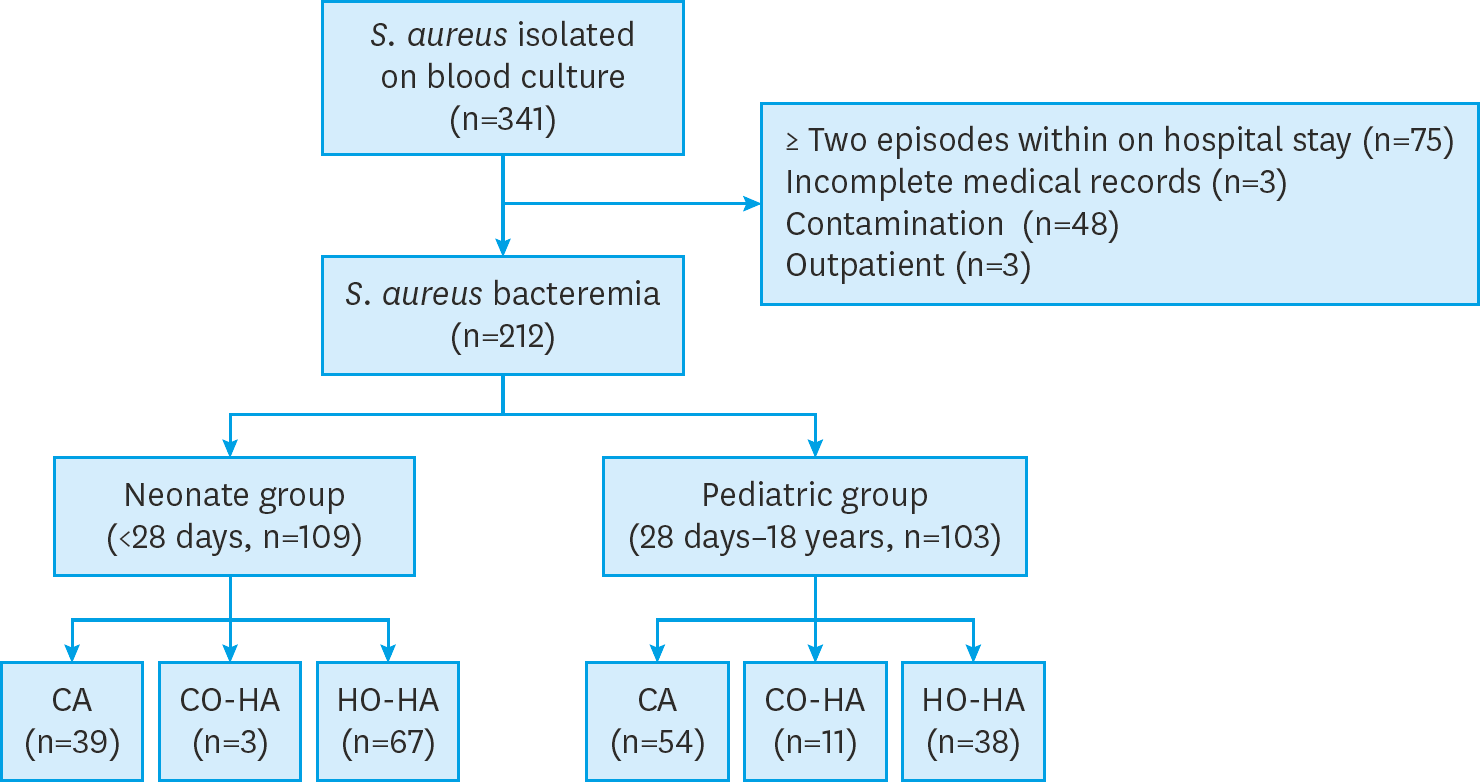 | Fig. 1.Schematic diagram to classify Staphylococcus aureus bacteremia cases included in the study. Abbreviations: CA, community associated; CO-HA, community onset-healthcare associated; HO-HA, hospital onset-healthcare associated. |
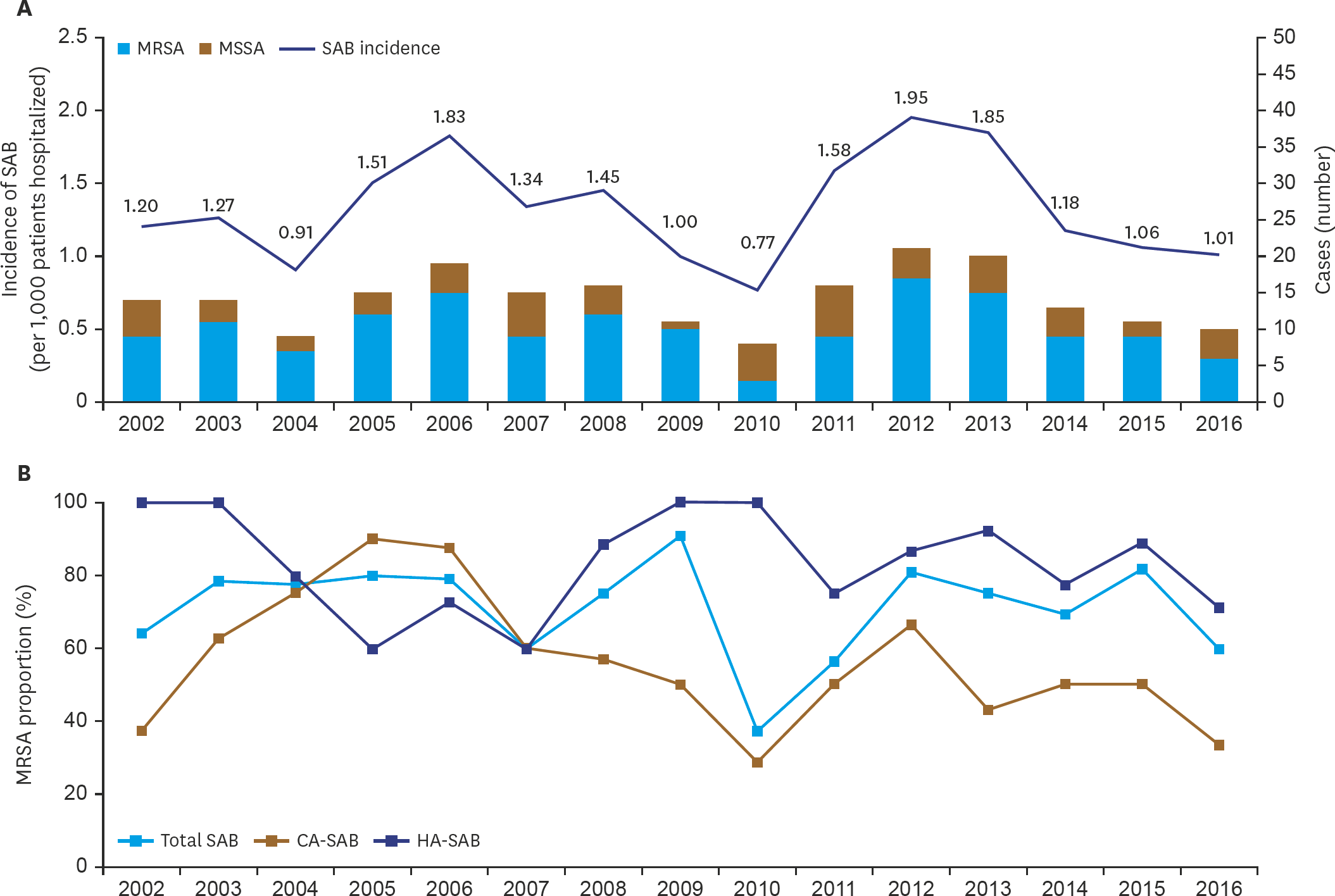 | Fig. 2.(A) The annual incidence of SAB and distribution of MSSA and MRSA among the SAB cases, (B) The annual MRSA proportion among the total SAB, CA-SAB, and HA-SAB cases. Abbreviation: SAB, Staphylococcus aureus bacteremia; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; CA-SAB, community-associated S. aureus bacteremia; HA-SAB, healthcare-associated S. aureus bacteremia; CA-MRSA, community-associated methicillin-resistant S. aureus; HA-MRSA, healthcare-associated methicillin-resistant S. aureus. |
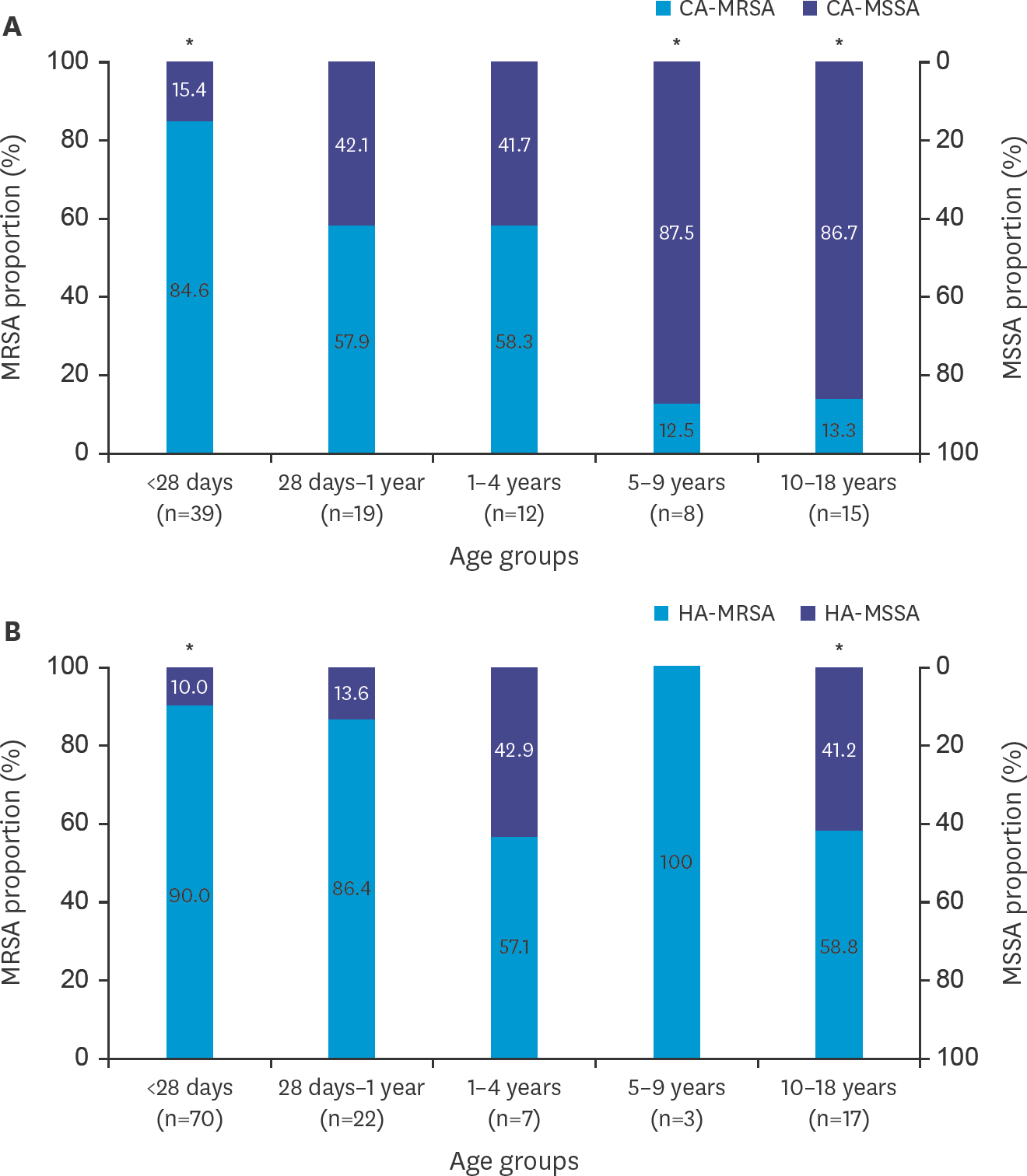 | Fig. 3.(A) Distribution of methicillin-resistance according to the age groups among CA-SAB. ORs in <28 days of age, 5–9 years of age, and 10–18 years of age are 8.64 (95% CI, 3.09–24.15; P=0.000), 0.09 (95% CI, 0.01–0.73; P=0.009), and 0.08 (95% CI, 0.02–0.37; P=0.000), respectively. (B) Distribution of methicillin-resistance according to the age groups among HA-SAB. ORs in <28 days group and 10–18 years group are 3.25 (95% CI, 1.19–8.89; P=0.018) and 0.21 (95% CI, 0.07–0.64; P=0.009), respectively. Abbreviations: CA-SAB, community-associated Staphylococcus aureus bacteremia; HA-SAB, healthcare-associated S. aureus bacteremia; CA-MRSA, community-associated methicillin-resistant S. aureus; CA-MSSA, community-associated methicillin-sensitive S. aureus; OR, odds ratio; CI, confidence interval. ∗P<0.05 by χ2 test. |
Table 1.
Baseline characteristics of SAB
| Characteristics Sex, male | Neonate group (n=109) 59 (54.1) | Pediatric group (n=103) 65 (63.1) | P-value 0.187 |
|---|---|---|---|
| Comorbid conditions | 58 (53.2)∗ | 28 (27.2)† | 0.000 |
| Central venous catheter | 43 (39.4) | 24 (23.3) | 0.011 |
| CA-SAB | 39 (35.8) | 54 (52.4) | 0.015 |
| HA-SAB | 70 (64.2) | 49 (47.6) | 0.015 |
| CO-HA | 3 (2.8) | 11 (10.7) | 0.020 |
| HO-HA | 67 (61.5) | 38 (36.9) | 0.000 |
| MRSA | 96 (88.1) | 57 (55.3) | 0.000 |
| Primary clinical manifestation | 18 (16.5) | 32 (31.1) | 0.012 |
| Bone and joint infections | 2 (14.3) | 13 (48.1) | 0.002 |
| Skin and soft tissue infections | 6 (42.9)‡ | 6 (22.2) | 0.920 |
| Septic thrombophlebitis | 6 (42.9)‡ | 0 | 0.016 |
| Pneumonia | 1 (7.1) | 4 (14.8) | 0.156 |
| Endocarditis | 0 | 2 (7.4)§ | 0.145 |
| Urinary tract infections | 1 (7.1) | 2 (7.4)§ | 0.530 |
| Peritonitis with urachal abscess | 0 | 1 (3.7) | 0.305 |
Abbreviations: SAB, Staphylococcus aureus bacteremia; CA, community associated; HA, healthcare associated; CO-HA, community onset-healthcare associated; HO-HA, hospital onset-healthcare associated; MRSA, methicillin-resistant S. aureus.
Table 2.
Complications and mortality of Staphylococcus aureus bacteremia according to the origin of infection
| Outcomes | CA (n=93) | HA (n=119) | P-value | |
|---|---|---|---|---|
| CO-HA (n=14) | HO-HA (n=105) | |||
| Complication | 3 (3.2) | 1 (7.1) | 5 (4.8) | 0.534∗ |
| Metastatic bone and joint infection | 3 (100.0) | 1 (100.0) | 3 (60.0) | 0.542∗ |
| Endocarditis | 0 | 0 | 2 (40.0) | – |
| Septic shock | 0 | 0 | 12 (11.4) | – |
| Mortality | 1 (1.1) | 1 (7.1) | 15 (14.3) | 0.003 |
Table 3.
Antimicrobial resistance and multidrug resistance of MRSA according to infection origin
| Antimicrobial agents | Total MRSA (n=153) | CA-MRSA (n=54) | HA-MRSA | P-value | |
|---|---|---|---|---|---|
| CO-HA MRSA (n=9) | HO-HA MRSA (n=90) | ||||
| Erythromycin | 92 (60.1) | 39 (72.2) | 5 (55.6) | 48 (53.3) | 0.078 |
| Clindamycin | 47 (30.7) | 16 (29.6) | 4 (44.4) | 27 (30.0) | 0.654 |
| Inducible resistance∗ | 26 (28.9)∗ | 13 (54.2)∗ | 0 | 13 (21.7)∗ | 0.124 |
| Gentamicin | 36 (23.5) | 14 (25.9) | 5 (55.6) | 17 (18.9) | 0.041 |
| Ciprofloxacin | 17 (11.1) | 2 (3.7) | 2 (22.2) | 13 (14.4) | 0.077 |
| Tetracycline | 52 (34.0) | 22 (40.7) | 4 (44.4) | 26 (28.9) | 0.275 |
| TMP-SMX | 4 (2.6) | 0 | 0 | 4 (4.4) | 0.450† |
| Vancomycin | 0 | 0 | 0 | 0 | – |
| MDR | 27 (17.6) | 9 (16.7) | 3 (33.3) | 15 (16.7) | 0.445 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; CA, community associated; HA, healthcare associated; CO-HA, community onset-healthcare associated; HO-HA, hospital onset-healthcare associated; TMP-SMX, trimethoprim-sulfamethoxazole; MDR, multidrug resistance




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download