Abstract
Purpose
This study aimed to investigate the relationship between uropathogens of infants with febrile urinary tract infection (UTI) and vesicoureteral reflux (VUR).
Materials and Methods
We analyzed 308 infants hospitalized for febrile UTI between January 2010 and December 2015, and assessed the voiding cystourethrography (VCUG). The medical records, including clinical symptoms, laboratory findings, urinalysis, urine culture tests, ultrasound (US), dimercaptosuccinic acid scan, and VCUG, were retrospectively obtained. The incidences of VUR and high-grade VURs (III, IV, and V) were analyzed in 4 groups categorized by uropathogens and renal US findings.
Results
The mean age of 308 infants was 3.29±2.18 months. The male-to-female ratio was 3.46:1. In urine culture tests, 267 infants (86.69%) showed single bacterial uropathogen; Escherichia coli in 241 infants (78.25%) and non-E. coli uropathogens in 26 infants (8.44%). Multiple distinctive microorganisms were identified as causative uropathogens in 41 infants (13.31%). Abnormal findings of US and VCUG were identified in 216 and 64 patients, respectively. In 308 infants, the incidences of VUR and high-grade VUR were not different among the 4 groups. In 239 male infants, the incidences of high-grade VUR were higher in patients with non-E. coli single or multiple uropathogen and with abnormal US findings (p=0.042).
Urinary tract infection (UTI) is a common infectious disease in infants, with a prevalence of 7.0%, presenting fever [1]. Vesicoureteral reflux (VUR) is one of the host risk factors that cause pediatric UTI. VUR has been identified in 30% to 50% of children with first UTI episode [2]. The prevalence of VUR can be estimated up to 70% in infants under the age of 12 months with UTI [3]. Most low-grade VURs (grades I and II) resolve spontaneously over time. However, this is not true in cases of high-grade VURs (III, IV, and V). The spontaneous resolution rate of grade III VUR is about 50% [4], and the rates of grades IV and V are as low as 9% to 25% [56].
While endoscopic treatment and surgical repair of VUR are successful with permanent correction rate, reaching 75% to 90% [78], undiagnosed or untreated VUR can lead to serious morbidities, such as hypertension and renal insufficiency, in children or young adults. Thus, appropriate detection and treatment of VUR in febrile infants with UTI appropriately are highly important. The gold standard for the diagnosis of VUR is voiding cystourethrography (VCUG). However, VCUG is a stressful procedure for infants and their parents. Moreover, it is an invasive study imposing risks of infection and radiation exposure. Several guidelines specify clinical conditions for VCUG in infants. The American Academy of Pediatrics guideline recommends a VCUG in children who are 2 to 24 months old presenting abnormal renal ultrasound (US) findings and/or atypical or recurrent UTI [9]. The National Institute for Health and Care Excellence guideline of United Kingdom recommends VCUG in infants younger than 6 months with abnormal renal US and/or atypical or recurrent UTI [10]. In Italy, the guidelines also include the condition of abnormal renal US findings for VCUG in infants [11].
As mentioned above, abnormal renal US findings are regarded as important clinical conditions when identifying VUR in infants with UTI. However, only a few previous studies have reported the relationship between various species of uropathogens, in addition to US findings and risk of VUR. In this study, we aimed to determine the difference of VUR and incidence of high-grade VURs (grades III, IV, and V) in infant groups with Escherichia coli as single uropathogen and non-E. coli uropathogen or between multiple uropathogens plus normal and abnormal renal US findings. Therefore, the relationship between uropathogens of infants w ith febrile UTI a nd VUR was i nvestig ated.
We identified 404 consecutive infant patients ≤12 months of age with febrile UTI who were admitted to Pusan National University Children's Hospital between January 2010 and December 2015. The study was approved by the local ethics committee of Pusan National University Yangsan Hospital (IRB No. 2017-068). The inclusion criteria were as follows: age ≤12 months, UTI documented on urine culture, body temperature at admission >37.5℃, and completion of renal US and VCUG tests. We retrieved data on age, sex, height, body weight, and temperature at the time of admission. The results of laboratory tests, including complete blood count test, liver function test, renal function test, and level of inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), were obtained. Patients' medical records were reviewed, and all data were acquired in a retrospective manner.
To evaluate urinary tract abnormalities, we conducted imaging examinations, including renal US, dimercaptosuccinic acid (DMSA) scan, and VCUG. The results of imaging tests before or 6 months after the admission date were used for analysis. The renal US tests were performed routinely in infants showing febrile UTI at the outpatient department or admission. Abnormal US findings were defined as follows: any grade of hydronephrosis, dilatation of ureter, thickened wall of renal pelvis, ureter or bladder, signs of acute pyelonephritis, and presence of ureterocele.
Indications of VCUG tests included recurrent or breakthrough UTI episodes during the follow-up period, presence of abnormal US findings, and presence of renal scar on DMSA scan. The presence and grade of VUR were documented based on the VCUG test. VUR grades III, IV, and V were considered as high-grade VURs. In cases of bilateral VUR, the higher grade of VUR was recorded.
Urine samples were routinely collected via clean catch midstream urine or bladder catheterization for routine urinalysis and urine culture test in all patients presenting febrile UTI. Pyuria was detected via urinalysis and defined as 6 or more neutrophils on high-power field of urine sample. Positive urine culture study was identified as a growth of bacteria by more than 105 colony-forming units. In cases of positive culture tests, subsequent antibiotics susceptibility tests were also performed. Negative culture study was excluded from analysis.
According to the results of renal US and urine culture test, we divided all patients into 4 groups: patients with E. coli as the single uropathogen with normal US findings (group 1); patients with E. coli as the single uropathogen with abnormal US findings (group 2); patients with single non-E. coli uropathogen or multiple uropathogens with normal US findings (group 3); and patients with single non-E. coli uropathogen or multiple uropathogens with abnormal US findings (group 4). We compared the VUR ratio and high-grade VUR ratio among the 4 groups. Same comparative analysis was performed in the subgroup of male infant patients. The subgroup analysis for female infant patients was not completed due to the small sample size of the female group.
We presented the patient characteristic variables as the mean±standard deviation in Table 1. The VUR ratio and high-grade VUR ratio were calculated and compared among the 4 groups via Pearson's chi-square test using categorical variables. All statistical analyses were performed using IBM SPSS Statistics ver. 20.0 software (IBM Co., Armonk, NY, USA).
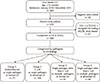
Of the 404 consecutive inpatients ≤12 months of age with febrile UTI between January 2010 and December 2015, 29 infants were confirmed to have negative urine culture studies and were excluded from analysis. The results of renal US and VCUG were not available in one patient and 62 patients, respectively. Moreover, we found that one patient had multicystic dysplastic kidney and 3 patients had renal stones on US findings. These patients were also excluded. A total of 308 infant patients were eligible for this study and formed the final study population for analysis (Fig. 1).
The patient characteristics of 308 infants are listed in Table 1. The mean age of the infants was 3.3±2.2 months. Among the 308 patients, 239 (77.6%) were male and 69 (22.4%) were female. The height, body weight, and body mass index were 77.7±14.2 cm, 11.2±4.3 kg, and 18.3±3.9 kg/cm2, respectively. The mean body temperature at admission was 38.7±0.7℃. Eighty-six infants (27.9%) presented body temperature≥39℃ when hospitalized.
On laboratory examinations, the mean level of white blood cells (WBC) was 15.7±6.1×103/µl. The mean segmented neutrophil was 47.6±13.5%. We calculated the level of inflammatory markers, such as CRP and ESR. The mean values of CRP and ESR were 4.9±4.6 mg/dl and 29.9±25.0 mm/hr, respectively. The laboratory values, such as WBC count, CRP, and ESR–related to infection or inflammation–were elevated higher than the upper normal limits. The level of segmented neutrophil percent is generally elevated in cases of infection; however, this was not true in our study.
DMSA scans were performed in 207 (67.2%) out of the 308 infants; about 53% of DMSA scans were identified as positive for renal cortical defects.
According to the results of routine urinalysis, 285 out of 308 (92.5%) patients showed pyuria. Based on urine culture data, we identified the number and species of causative microorganisms for febrile UTI. Of the 308 patients, 267 infants (86.7%) suffered UTI caused by single bacterial uropathogen; E. coli as the single uropathogen in 241 out of 308 cases (78.3%) and non-E. coli uropathogen in 26 of 308 cases (8.4%). Moreover, 2 and 3 distinctive microorganisms were identified as causative uropathogens in 37 (12.0%) and 4 patients (1.3%), respectively (Table 1).
The urine culture findings of 308 febrile UTI patients are listed in Table 2. A total of 353 uropathogens were isolated from urine culture in 308 infants. E. coli was identified as the most common uropathogen in 279 of 353 isolates (79.0%), followed by Klebsiella spp., Enterococcus spp., Enterobacter spp., Citrobacter spp., and other species (25/353, 7.1%; 24/353, 6.8%; 11/353, 3.1%; 6/353, 1.7%; 8/353, 2.3%; respectively). Klebsiella spp. included 17 Klebsiella pneumonia strains and 8 Klebsiella oxytoca strains. Other microorganism species included were as follows: 2 Proteus spp., 2 Streptococcus agalactiae, 1 Pseudomonas spp., 1 Staphylococcus aureus, 1 Raoultella planticola, and 1 Morganella morganii.
The incidences of VUR by grade are presented in Table 3. Of the 308 infants with febrile UTI, 64 (20.8%) showed positive findings on VCUG. Finally, 42 infants (13.6%) were proved to have high-grade VURs (grades III, IV, and V).
We categorized 308 patients by uropathogens and US findings into 4 groups and compared the ratio of VUR and high-grade V UR between the groups. The comparative analysis of VUR and high-grade VUR ratio is shown in Tables 4 and 5, respectively. In 308 patients, VUR and high-grade VUR ratio were not different between the groups with normal and abnormal US findings (p=0.206 and 0.361, respectively). In the group with normal US findings, no difference in VUR and high-grade VUR ratio was found between E. coli and non-E. coli patients (p=0.292 and 0.275, respectively). Moreover, in the group with abnormal US findings, no difference was noted (p=0.129 and 0.059, respectively).
We compared the VUR and high-grade VUR ratio via Pearson's chi-square test in the subgroup of male infant patients. A total of 239 male infants were divided into 4 groups in the same manner described above. There were 48, 130, 1 7, a nd 4 4 patients in group 5 ( E. coli as the single uropathogen with normal US findings), group 6 (E. coli as the single uropathogen with abnormal US findings), group 7 ( sing le n on-E. coli u ropathog en o r multiple uropathogens with normal US findings), and group 8 (single non-E. coli uropathogen or multiple uropathogens with abnormal US findings), respectively. The results of VUR and high-grade VUR ratio analysis are presented in Tables 6 and 7, respectively. In the group with abnormal US findings, the high-grade VUR ratio was higher in the non-E. coli group than in the E. coil group (p=0.041).
We also analyzed the incidences of renal scars on DMSA scan. A total of 204 infants completed the DMSA scans. We observed more cortical defects in the infant group showing abnormal US findings or VUR (p=0.013 and 0.038, respectively). The results are listed in Table 8. There were no differences in the cortical defect incidence by uropathogens.
We aimed to investigate the incidences of VUR and high-grade VUR in 4 infant groups with E. coli as the single uropathogen vs. non-E. coli uropathogen or multiple uropathogens, in addition to between the normal and abnormal renal US findings. In male infants showing abnormal US findings, high-grade VUR incidences were higher in the non-E. coli uropathogen group than in the E. coli group.
Renal US i s a mainstay modality of r enal imag ing for the management of VUR in children. The presence and severity of hydronephrosis can be estimated through ante- and postnatal US. Renal US also helps clinicians to follow-up with the renal growth over time and make critical decisions on VUR management. Moreover, US is an easy, noninvasive imaging modality without radiation exposure. Nevertheless, renal US cannot reliably rule out the presence of VUR by itself. Farhat et al. [12] demonstrated that postnatal US findings had poor correlations with the presence and severity of VUR, suggesting to not exclude VCUG in normal US cases to diagnose VUR. In a study of 144 children under 2 years of age presenting febrile UTI, Massanyi et al. [13] showed that the sensitivity and negative predictive value of US for renal abnormalities were only 16% and 25%, respectively. Even in grades IV and V VUR, the sensitivity of US was only 36%. Wongbencharat et al. [14] showed that in 387 infants under one year of age with f irst febrile UTI, the sensitivity of renal US and late 6 month DMSA scan in detecting grade IV and V VURs were 50% and 87.5%, respectively. After reviewing several studies, we found that renal US alone had a very limited role, with relatively low sensitivity, in predicting VUR and high-grade VUR in children with febrile UTI. In our study, the sensitivities of renal US for VUR and high-grade VUR were 76.6% (49/64) and 73.8% (31/42), respectively. The values of sensitivity were higher than those in other studies. In the infant group without VUR, 68.4% showed positive US finding (167/244).
In addition to abnormal US findings, some studies reported the association of non-E. coli UTI and VUR. Ristola et al. [15] showed that in 282 patients younger than 3 years with febrile UTI, abnormal US finding, atypical infection, non-E. coli infection, and recurrent infection were risk factors for VUR. They also identified that non-E. coli infection was the only factor that predicted abnormal US finding, recommending continuous follow-up without further imaging in children with no risk factors for VUR. Friedman et al. [16] demonstrated that non-E. coli strain was associated with urinary tract anomalies, such as grade III and IV VURs, hydronephrosis, and ureteropelvic junction obstruction. Choi et al. [17] investigated risk factors for g rade I II and IV VURs in 446 p atients with f ebrile UTI. They found that non-E. coli UTI, in addition to recurrent UTI, abnormal US, and DMSA scan findings, was associated with grade IV and V VURs. Pauchard et al. [18] showed that clinicians could avoid VCUG in infants ≤3 months with first febrile UTI by E. coli and normal renal findings at a low risk ≤1% of missing high-grade VURs (grades III, IV, and V). When the abnormal US findings were added, the detection probability of high-grade VUR increased from 26% to 55% in the non-E. coli infection group.
In our study, we took US findings and species of uropathogens together into consideration when estimating VUR incidences. In male infants showing abnormal US findings, we demonstrated a higher ratio of high-grade VUR in the non-E. coli group than in the E. coli group. Among the male infants with abnormal US findings, a much higher ratio of high-grade VURs (15.9%) than low-grade VURs (0.0%) was shown in the non-E. coli group. In the E. coli group, however, the ratio of high-grade VURs and low-grade VURs was the same (13.1%, 13.1%, respectively). This indicates that in the presence of VUR, non-E. coli uropathogens male infants with abnormal US findings have high-grade VUR with high probability. Nevertheless, there are no differences in the ratio of high-grade VURs between the non-E. coli (15.9%) and E. coli (13.1%) group (p>0.05). Moreover, in a total of 308 infants and in male infants with normal US findings, the ratios of VUR or high-grade VUR were not d ifferent by the species of u ropathogens.
This study has some limitations. First, we did not consider the circumcision status in male infants. Hayashi and Kohri [19] presented that circumcision may offer protection against UTI in male newborns. In addition, Herndon et al. [20] reported significantly higher incidences of breakthrough UTI in uncircumcised boys with VUR than in circumcised counterparts (53% vs. 19%). In our study, the effects of circumcision status on UTI occurrence in male infants with VUR was not estimated, because circumcision in infancy is not common in Korean culture. Therefore, enrolling circumcised male infants in this type of research was difficult. Second, male infants far outnumbered female infants in our study. The ratios of male to female infants were 77.6:22.4, respectively. The results of VUR incidence analysis in 308 patients, including boys and girls, could not be generalized in female patients. Due to small sample size, analysis of the female subgroup was not possible.
Figures and Tables
Fig. 1
Flowchart of patient inclusion. UTI: urogential tract infection, US: ultrasound, VCUG: voiding cystourethrography, MCDK: multicystic dysplastic kidney.
ACKNOWLEDGMENTS
This study was supported by a 2017 research grant from Pusan National University Yangsan Hospital.
References
1. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008; 27:302–308.
2. Greenfield SP, Wan J. Vesicoureteral reflux: practical aspects of evaluation and management. Pediatr Nephrol. 1996; 10:789–794.

3. Baker R, Maxted W, Maylath J, Shuman I. Relation of age, sex and infection to reflux: data indicating high spontaneous cure rate in pediatric patients. J Urol. 1966; 95:27–32.

5. Weiss R, Tamminen-Mobius T, Koskimies O, Olbing H, Smellie JM, Hirche H, et al. The International Reflux Study In Children. Characteristics at entry of children with severe primary vesicoureteral reflux recruited for a multicenter, international therapeutic trial comparing medical and surgical management. J Urol. 1992; 148:1644–1649.

6. Skoog SJ, Belman AB, Majd M. A nonsurgical approach to the management of primary vesicoureteral reflux. J Urol. 1987; 138:941–946.

7. O'Donnell B, Puri P. Endoscopic correction of primary vesicoureteric reflux. Br J Urol. 1986; 58:601–604.
8. Kirsch AJ, Perez-Brayfield M, Smith EA, Scherz HC. The modified sting procedure to correct vesicoureteral reflux: improved results with submucosal implantation within the intramural ureter. J Urol. 2004; 171:2413–2416.

9. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2–24 months of age. Pediatrics. 2016; 138:e20163026.
10. Mori R, Lakhanpaul M, Verrier-Jones K. Diagnosis and management of urinary tract infection in children: summary of NICE guidance. BMJ. 2007; 335:395–397.

11. Ammenti A, Cataldi L, Chimenz R, Fanos V, La Manna A, Marra G, et al. Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow-up. Acta Paediatr. 2012; 101:451–457.

12. Farhat W, McLorie G, Geary D, Capolicchio G, Bagli D, Merguerian P, et al. The natural history of neonatal vesicoureteral reflux associated with antenatal hydronephrosis. J Urol. 2000; 164:1057–1060.

13. Massanyi EZ, Preece J, Gupta A, Lin SM, Wang MH. Utility of screening ultrasound after first febrile UTI among patients with clinically significant vesicoureteral reflux. Urology. 2013; 82:905–909.

14. Wongbencharat K, Tongpenyai Y, Na-Rungsri K. Renal ultrasound and DMSA screening for high-grade vesicoureteral reflux. Pediatr Int. 2016; 58:214–218.

15. Ristola MT, Loyttyniemi E, Hurme T. Factors associated with abnormal imaging and infection recurrence after a first febrile urinary tract infection in children. Eur J Pediatr Surg. 2017; 27:142–149.

16. Friedman S, Reif S, Assia A, Mishaal R, Levy I. Clinical and laboratory characteristics of non-E. coli urinary tract infections. Arch Dis Child. 2006; 91:845–846.

17. Choi EJ, Lee MJ, Park SA, Lee OK. Predictors of high-grade vesicoureteral reflux in children with febrile urinary tract infections. Child Kidney Dis. 2017; 21:136–141.

18. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017; 102:804–808.





 PDF
PDF ePub
ePub Citation
Citation Print
Print











 XML Download
XML Download