Abstract
Background
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited small vessel disease caused by mutations in the Notch3 gene. Lacunes may reflect occlusive type microangiopathy. However, cerebral microbleeds (CMBs) may reflect bleeding-prone microangiopathy. In the present study, we aimed to determine whether hypertension influence the distribution and severity of lacunes and CMBs in patients with CADASIL.
Methods
The study population comprised 85 patients who underwent brain MRI, including T1-weighted image, susceptibility weighted image (SWI), and fluid attenuated inversion recovery (FLAIR) image. The patients were divided into two groups depending on the presence or absence of hypertension. In the first, demographic factors, and MRI findings were compared between CADASIL patients with and without hypertension. In the second, we undertook a region by region comparison of number of patients with lacunes or CMBs.
Results
The hypertensive group showed a higher incidence of CMBs in lobar area (p<0.001) and basal ganglia (p=0.014). CMBs tend to be observed more frequently in the thalamus (p=0.058), brainstem (p=0.057), and cerebellum (p=0.052) in the hypertensive group. However, hypertensive group demonstrated a higher incidence of lacunes just in lobar area (p=0.040).
Figures and Tables
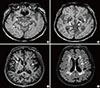 | Fig. 1A 63 year-old man who had one stroke (mild left-sided weakness). Susceptibility Weighted Image (SWI) showing multiple CMBs in brain (A, B). 3D-T1 weighted image and Fluid-attenuated inversion recovery (FLAIR) image showing multiple lacunes in brain (C, D). Arrowhead indicates cerebral microbleeds. Arrow points to lacunes. |
Table 1
Demographics and clinical characteristics of CADASIL patients with or without HTN

Comparison: independent T test for age and educational level, Chi-square test for the other variables. Data are mean±SD or n (%) values.
*p<0.05; **p<0.01; ***p<0.001.
CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; HTN, hypertension; CMBs, cerebral microbleeds.
References
1. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch 3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996; 383:707–710.
2. Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol. 2009; 8:643–653.
3. Lee JS, Choi JC, Kang SY, Song SK, Kang JH, Song JK, et al. Clinical and MRI profiles predicting clinical overt stroke in patients with CADASIL. J Korean Neurol Assoc. 2012; 30:93–99.
4. Lee SH, Kwon SJ, Kim KS, Yoon BW, Roh JK. Cerebral microbleeds in patients with hypertensive stroke: topographical distribution in the supratentorial area. J Neurol. 2004; 251:1183–1189.
5. Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006; 129:2375–2383.

6. Choi JC, Song SK, Lee JS, Kang SY, Kang JH. Diversity of stroke presentation in CADASIL: study from patients harboring the predominant NOTCH3 mutation R544C. J Stroke Cerebrovasc Dis. 2013; 22:126–131.

7. Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction form enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998; 245:116–122.

8. Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006; 66:165–171.

9. Lesnik Oberstein SA, van den, van Buchem MA, van Houwellingen HC, Bakker E, Vollebregt E, et al. Cerebral microbleeds in CADASIL. Neurology. 2001; 57:1066–1070.

10. Kim Y, Choi EJ, Choi CG, Kim G, Choi JH, Yoo HW, et al. Characteristics of CADASIL in Korea: a novel cysteine-sparing Notch3 mutation. Neurology. 2006; 66:1511–1516.

11. Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006; 129:2375–2383.

12. Lee JS, Chio JC, Kang SY, Na HR, Kang JH. Vascular risk factors in CADASIL patients with Notch R544C mutation. Dement Neurocogn Disord. 2009; 8:98–103.

13. Lee JS, Park SW, Song SK, Choi JC, Kang SY, Kang JH. The association between hypertension and cerebral microbleeds in patients with CADASIL. J Korean Neurol Assoc. 2014; 32:82–87.

14. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010; 9:689–701.

15. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009; 8:165–174.

16. Lee SH, Bae HJ, Ko SB, Kim H, Yoon BW, Roh JK. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry. 2004; 75:423–427.

17. Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am J Neuroradiol. 2009; 30:338–343.

18. Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM. Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging. 2010; 31:142–148.

19. Razvi SS, Davidson R, Bone I, Muir KW. The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in the west of Scotland. J Neurol Neurosurg Psychiatry. 2005; 76:739–741.

20. Lee JS, Choi JC, Kang SY, Kang JH, Lee SH, Kim JH, et al. Olfactory identification deficits in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Eur Neurol. 2010; 64:280–285.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download