Abstract
Vertebral artery dissection is one of the most common causes of stroke in young adults. The course of the vertebral artery dissection is usually benign, and pure transient amnesia as an initial symptom has been rarely reported. We describe a patient with vertebral artery dissection who presented with acute transient amnesia, and review the medical literatures about the pathophysiological mechanism of transient global amenesia (TGA). This case could be a one of evidence which supports the cerebrovascular mechanism of TGA.
Vertebral artery dissection is one of the most common causes of stroke in young adults [1]. The course of the vertebral artery dissection is usually benign, but seldom reaches to severe complication, such as brainstem infarction, subarachnoid hemorrhage (SAH) or death [2]. The clinical manifestation includes headache, cervical pain, dizziness or cranial nerve palsy [2]. However, pure transient amnesia as an initial symptom has been rarely reported in those patients.
The body of the hippocampus is mainly supplied by the longitudinal terminal segments of the hippocampal arteries which are branched from the anterior, middle, and posterior hippocampal artery of posterior cerebral artery [3]. Therefore, thromboembolism from vertebral artery dissecting wall hematoma or lumen might cause the CA 1 sector of hippocampus infarction.
Herein, we describe a patient with vertebral artery dissection who presented with acute transient amnesia with prolonged cognitive decline and review the medical literatures about the pathophysiological mechanism of transient global amenesia (TGA).
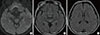
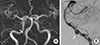
A 58-year-old man visited our Memory Clinic with complaints of memory decline during landscaping work since nine weeks ago. He was sawing wood for landscape on tall tree, and after several hours' work, he repeated to ask same questions. His colleagues reported that the patient asked stereotypic questions, repeatedly, while he was able to use the mechanical tools and to drive home from the workplace. The attack has disappeared after two or three hours, and he denied the neck pain, dizziness or transient visual loss around that period. Although his anterograde global amnesia has disappeared, the patient complained of subtle cognitive decline after the event. The patient was not able to memorize the telephone numbers or felt difficulty to find the place where he put his usual objects. Physical examination was unremarkable and neurological examination revealed no abnormal findings. The neuropsychological test showed deficits in memory retrieval and mild frontal dysfunction. TGA with prolonged cognitive decline was diagnosed and brain magnetic resonance imaging (MRI) was assessed to evaluate the cause of cognitive decline including stroke. The MR images were obtained at Konkuk University Medical Center using a Signa HDx 3.0 Tesla unit (GE Healthcare, Milwaukee, WI, USA) with an 8-channel high-resolution head coil. The MRI protocol included the following sequences: 1) The axial diffusion-weighted [b factor of 2,000 s/mm2, TR/TE, 1,000/75 ms, section thickness, 3.5 mm; matrix, 128×128; Field of view (FOV), 240×240 mm; Gap, 0]; 2) axial T2-weighted fast spin-echo (TR/effective TE, 4,000/106 ms; section thickness, 5 mm; matrix 384×384); and 3) axial fluid-attenuated inversion-recovery (FLAIR) (TR/TE/TI, 11,000/105/2,600 ms; section thickness, 5 mm; matrix, 384×224). Brain diffusion-weighted and FLAIR images showed no abnormal findings (Fig. 1), but magnetic resonance angiography demonstrated a fusiform dilatation in the right vertebral artery suggesting vertebral arterial dissection (VAD). The conventional angiography was conducted, showing an unruptured, fusiform dissecting aneurysm at the right vertebral artery (Fig. 2). TGA with prolonged cognitive decline due to VAD was diagnosed. Predisposing risk factors of the extracranial dissection, including fibromuscular dysplasia, periarteritis nodosa, syphilitic angiopathy, artherosclerosis, Marfan's syndrome or Ehlers-Danloss syndrome were excluded. TGA at nine weeks ago and long-lasting neuropsychological deficit was diagnosed. His memory ability gradually improved over 8 weeks, and the result of follow-up neuropsychological evaluation showed within normal limits including memory test. Antiplatelet therapy was kept for the 1 year and then discarded.
The patient had TGA events, but had more prolonged cognitive decline for about 17 weeks. It could be an unusual symptom that the patient had mild deficit in memory retrieval and frontal function. However, this has been reported in many studies that mild cognitive decline could be prolonged for certain times after the TGA events [4, 5, 6]. The meta-analysis study also showed that the reductions in both anterograde episodic long-term memory and executive function recover slowly, as poor cognitive performance of TGA patients continued for weeks or months after the attack [7]. Merely, the temporal correlation of VAD with TGA could be uncertain, whether VAD was only related with cognitive decline after TGA attack or also could be considered as the cause of TGA, because the evaluation was assessed since 9 weeks after the TGA attack. We considered the VAD was also the case of TGA and prolonged cognitive decline after the attack, because the TGA and VAD both have characteristics of "sudden onset". Moreover, the patient was working on tall tree which is possibly consisted of a vigorous neck movement at the time of event. As we diagnosed the patient had TGA with cognitive decline due to VAD, we reviewed the pathophysiology of TGA.
TGA is a well-defined clinical syndrome, which defined by an aneterograde and retrograde amnesia of sudden onset that lasts up to 24 hours. Despite of clear clinical criteria, the pathophysiology is perplexed [8]. The transient dysfunction of the hippocampus, especially Cornus Ammnonis 1 (CA 1) sector is known as the main mechanism. Although many factors such as migraine, hypoxic-ischemic events, venous congestion and epilepsy-related activities contribute to the dysfunction of CA 1 sector, it is not revealed that which is the leading stage.
Cerebrovascular events are one of the most powerful one because of its abrupt onset and anatomical lesion. The radiant development of radiology improved the imaging detection rate of TGA lesions up to 88% with modified MRI diffusion-weighted imaging (DWI) protocol [9]. Most of imaging data revealed an involvement of memory circuits in TGA, which present high signal intensity at DWI correlating to restricted apparent diffusion coefficient [9, 10, 11]. In the circuit, many reports points out the CA 1 sector as a critical lesion of TGA [10, 12]. CA 1 sector is in the watershed area of internal anastomosis between upper and lower hippocampal arteries, and the lesion is vulnerable to haemodynamic change [3]. Furthermore, Clinical and experimental data show that neurons of CA 1 sector are selectively vulnerable to hypoxemia and ischemia which cause glutmate- and calcium-induced neuronal death of affected neurons [13]. Previous report showed that TGA patients had a significantly higher prevalence of some cardiovascular risk factors, such as hyperlipidemia and ischemic heart disease [14]. However, several case-control studies and meta-analysis did not find any association between vascular risk factors and TGA, including migraine [10].
Migraine with aura is one of the risk factor of TGA and migrainer has a higher incidence of TGA than healthy controls [15, 16]. Cortical spreading depression which is transient neuronal depolarization followed by a long-lasting hypoperfusion and suppression of neuronal activity explain the mechanism of migraine, and it applied to TGA. The following hypoperfusion and metabolic suppression might affect hippocampus either, and represented as transient memory loss [8]. Because CA 1 sector has many inotropic glutamate receptors, CA 1 sector have the selective vulnerability to neuronal depolarization [13]. Migraine release the glutamate which trigger the spreading depression, also emotional event which is a precipitating factors of TGA also lead to glutamate release. This glutamate-related neuronal suppression is one of the attractive mechanism that can explain the TGA, however meta-analysis study reports no association with migraine and TGA [10].
Increased venous pressure which associated with Valsalva maneuvers in TGA change the flow patterns of jugular vein to retrograde. TGA patients have high rate insufficient jugular vein valves compared with healthy controls, it is confirmed by many imaging study [17, 18]. Some embolisms which are not strained through jugular vein valve during increase in intracranial venous pressure can make focal hippocampal lesions. However, a correlation between intracranial venous drainage and jugular valve insufficiency was not found in ultrasonography and magnetic resonance venography studies [19].
Vertebral artery dissection is common cause of young age stroke. The most common dissecting part is the V3 segment of the vertebral artery, where is some greater mobility of the extracranial arteries may increase the potential to be damaged by cervical vertebrae or styloid process [2]. Vertebral artery dissection can advance to cerebral infarction of posterior circulation territory through vessel wall hematoma, intraluminal thrombus, or subarachnoid hemorrhage [20]. Our case emphasizes that the acute transient global amnesia with prolonged cognitive impairment could be a clue for secondary TGA like a vertebral artery dissection and angiographic evaluation should be considered even in patients without evidence of brain parenchymal lesion. Furthermore, this case could be a one of evidence which supports the cerebrovascular mechanism of TGA, however still further studies are needed.
Figures and Tables
References
1. Lee VH, Brown RD Jr, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: A population-based study. Neurology. 2006; 67:1809–1812.

2. Debette S, Leys D. Cervical-artery dissections: Predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009; 8:668–678.

3. Sedlaczek O, Hirsch JG, Grips E, Peters CN, Gass A, Wohrle J, et al. Detection of delayed focal MR changes in the lateral hippocampus in transient global amnesia. Neurology. 2004; 62:2165–2170.

4. Lampl Y, Sadeh M, Lorberboym M. Transient global amnesia-not always a benign process. Acta Neurol Scand. 2004; 110:75–79.

5. Guillery-Girard B, Quinette P, Desgranges B, Piolino P, Viader F, de la Sayette V, et al. Long-term memory following transient global amnesia: An investigation of episodic and semantic memory. Acta Neurol Scand. 2006; 114:329–333.

6. Le Pira F, Giuffrida S, Maci T, Reggio E, Zappala G, Perciavalle V. Cognitive findings after transient global amnesia: Role of prefrontal cortex. Appl Neuropsychol. 2005; 12:212–217.

7. Jager T, Bazner H, Kliegel M, Szabo K, Hennerici MG. The transience and nature of cognitive impairments in transient global amnesia: A meta-analysis. J Clin Exp Neuropsychol. 2009; 31:8–19.

8. Bartsch T, Deuschl G. Transient global amnesia: Functional anatomy and clinical implications. Lancet Neurol. 2010; 9:205–214.

9. Kim J, Kwon Y, Yang Y, Jang IM, Chang Y, Park YH, et al. Clinical experience of modified diffusion-weighted imaging protocol for lesion detection in transient global amnesia: An 8-year large-scale clinical study. J Neuroimaging. 2013.

10. Bartsch T, Alfke K, Stingele R, Rohr A, Freitag-Wolf S, Jansen O, et al. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 2006; 129:2874–2884.

11. Pearce JM, Bogousslavsky J. 'Les ictus amnesiques' and transient global amnesia. Eur Neurol. 2009; 62:188–192.
12. Bartsch T, Alfke K, Deuschl G, Jansen O. Evolution of hippocampal CA-1 diffusion lesions in transient global amnesia. Ann Neurol. 2007; 62:475–480.

13. Milusheva EA, Baranyi M. Implication of ionotropic glutamate receptors in the release of noradrenaline in hippocampal CA1 and CA3 subregions under oxygen and glucose deprivation. Neurochem Int. 2003; 43:543–550.

14. Jang JW, Park SY, Hong JH, Park YH, Kim JE, Kim S. Different risk factor profiles between transient global amnesia and transient ischemic attack: A large case-control study. Eur Neurol. 2014; 71:19–24.

15. Hodges JR, Warlow CP. Syndromes of transient amnesia: Towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990; 53:834–843.

16. Pantoni L, Bertini E, Lamassa M, Pracucci G, Inzitari D. Clinical features, risk factors, and prognosis in transient global amnesia: A follow-up study. Eur J Neurol. 2005; 12:350–356.

17. Chung CP, Hsu HY, Chao AC, Chang FC, Sheng WY, Hu HH. Detection of intracranial venous reflux in patients of transient global amnesia. Neurology. 2006; 66:1873–1877.

18. Akkawi NM, Agosti C, Borroni B, Padovani A. Detection of intracranial venous reflux in patients of transient global amnesia. Neurology. 2007; 68:163. author reply 163.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download