Abstract
Nelumbo nucifera Gaertn. (Nymphaeaceae) is commonly called lotus and its leaves are widely been used as functional ingredients due to its antioxidant activity. For maximum efficacy, optimized extraction condition was established using response surface methodology. The high F-values, low p-values and insignificant p-value for lack-of-fit supported the fitness of the model and yielded the second-order polynomial regression for the antioxidant activity. The optimized extract was obtained by the extraction of 1 g of lotus leaves with 40 mL of 50% MeOH at 10.0℃, which exerted 70.1% antioxidant activity. Close correlation between phenolic content and antioxidant activity suggested phenolic compounds as active constituents of lotus leaves. In addition, comparison of different parts of lotus demonstrated the most potent antioxidant activity of flowers, followed by leaves and roots. Taken together, these results provide useful information about lotus leaves for the development as antioxidant ingredients. In addition, flowers and roots as well as leaves are suggested as good sources for antioxidant activity.
Nelumbo nucifera Gaertn. (Nymphaeaceae), commonly called lotus, is a perennial aquatic herb that is widely distributed throughout Eastern Asia. Lotus leaves are used for tea and are commercially available in Korea. Steamed rice wrapped with lotus leaves are also consumed as healthy foods for its diverse activity. Other parts of the lotus such as the flowers, seeds and roots are available and have been used in traditional medicine for a long time. Therefore, lotus is widely cultivated and consumed as food and medicine in Korea. Previous studies have revealed the pharmacological effects of lotus leaves, including antioxidant,12 anti-diabetic3 and anti-obesity.45 Flavonoids and benzylisoquinoline alkaloids have been reported from lotus leaves.678 Our previous study reported megastigmanes, flavonoid, alkaloids and flavolignans from the lotus leaves and their inhibitory effect on pancreatic lipase.7
Oxidative stress derives from an imbalance between the production of reactive oxygen species and antioxidant defense. It is well known as major contributor to diverse diseases such as cancer, inflammation, neurodegenerative diseases and diabetes.91011 It also affects age-related symptoms such as fatigue, skin damage and senescence. Antioxidant defense system in our body such as superoxide dismutase, catalase and glutathione reduces reactive oxygen stress (ROS) protects our body from oxidative stress. Natural products with high antioxidant ingredients are also beneficial for reducing oxidative stress.121314
Lotus is well known for antioxidant effect and used as functional ingredients.12151617 For development of products, optimization of extraction condition is required for maximum efficacy. During extraction, many extraction factors such as extraction solvent, extraction time, extraction temperature and solid-liquid ratios affect the composition of extract as well as its biological activity.181920 Response surface methodology (RSM) is a statistical tool and takes into several factors simultaneously using rationally designed experiment. Therefore, optimal condition can be effectively derived especially in case of several variables.212223
In the present study, antioxidant activity of lotus leaves was investigated using DPPH radical scavenging assay system and optimized extraction condition was derived using RSM with three-level-three-factor Box-Behnken design (BBD). The correlation between activities and phenolic content was also analyzed. Since different plant parts may contain different types of constituents which contribute to the biological activities,24252627 the antioxidant activities of different parts of lotus were also compared.
Leaves, flowers, seeds and roots of N. nucifera were prepared from the local herbal market in Chungbuk, Korea in January 2016. They were identified by the herbarium of the College of Pharmacy at Chungbuk National University, where voucher specimens were deposited (CBNU201601-NNL, NNF, NNS and NNR). Leaves, flowers, seeds and roots of lotus were extracted twice with 80% MeOH for the evaluation of antioxidant activity and phenolic content.
RSM with three variables and three levels BBD was used to optimize the extraction conditions of lotus leaves for antioxidant activity. In the present study, extraction solvent, MeOH (X1), extraction temperature (X2) and solvent volume / dried lotus leaves (X3) were chosen as three independent extraction variables. After determination of the ranges of these variables on the basis of a preliminary single factor experiment, complete design with 15 experimental points including three replication of the center points (all variables were coded as zero) was set up.
The fitness of the polynomial model equation to the responses was evaluated with the coefficients of R2 and the lack of fit was evaluated using F-test.
The antioxidant activity was evaluated by measuring the free radical scavenging activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH). The radical scavenging activity was determined by measuring the absorbance at 517 nm. The relative radical scavenging activity (%) was calculated as [1 − absorbance of solution with sample and DPPH / absorbance of solution with DPPH] × 100.
The total phenolic content was measured with a Folin-Ciocalteau assay. Folin-Ciocalteau's phenol reagent was added to the 96-well plate containing the test samples. After 5 min of incubation with gentle shaking, 7% Na2CO3 was added to the reaction mixture. The reaction mixture was left in the dark for 90 min at room temperature. The absorbance was measured at 630 nm with a microplate reader. The total phenolic content was expressed as gallic acid equivalent (GAE) using gallic acid as a standard.
We first investigated the antioxidant activity of extract of lotus leaves using DPPH radical scavenging assay. The total extract of lotus leaves showed dose-dependent antioxidant activity with IC50 values of 100.2 µg/mL. We previously investigated the constituents of lotus leaves and reported thirty two compounds such as megastigmanes, alkaloids, flavonoids and phenolic compounds.7 Therefore, we next investigated the antioxidant activity of the constituents of lotus leaves. Four constituents were selected from each skeleton such as vomifoliol (1), a megastigmane, roemerine-N-oxide (2), an alkaloid, quercetin (3), a flavonoid and trans-N-feruloyltyramine (4), a phenolic compound (Fig. 1). Among them, quercetin (3) and trans-N-feruloyltyramine (4) exerted antioxidant activity with IC50 values of 6.6 and 69.4 µg/mL, respectively. Vomifoliol (1) and roemerine-N-oxide (2), however, showed weak activity with IC50 values of >100 µg/mL. These results suggested that phenolic compounds contribute the antioxidant activity of lotus leaves.
Extraction conditions greatly affect the biological activity as well as the constituents of extract.181920 Therefore, effect of extraction condition of lotus leaves on antioxidant activity and phenolic content was investigated. In addition, extraction conditions of lotus leaves was optimized for maximum antioxidant activity using RSM employing BBD with three-level-three-factor (Table 1).
Extraction solvent, extraction temperature and solvent/sample ratio were chosen for extraction variables and the ranges of each variables were selected as extraction solvent (X1, MeOH concentration in water, 0, 50 and 100%), extraction temperature (X2, 10, 40 and 70℃) and solvent/sample ratio (X3, 10, 30 and 50) on the preliminary single factor experiment. Antioxidant activities were varied from 43.4 to 70.4% depending on of each experimental point, which showed the importance of extraction condition for antioxidant activity.
Second-order polynomial regression equation was established by RSM for the evaluation of the relationship between variables and responses. Greater coefficients with smaller p-value (p<0.05) indicated the considerable effect of these coefficients on response (Table 2). The value of multiple determination (R2) was 0.946, which demonstrated effectiveness of this model. Insignificant p-value of lack of fit, 0.767 also indicated the adaptability of this model to experimental data (Table 3). Relationship between every two variables for antioxidant activity was also shown in three dimensional response surface plots based on regression equations (Fig. 2). Collectively, this model is adequately fitted to the experimental data and suitable for optimization.
Multiple regression analysis on the experimental data yielded the second-order polynomial regression equation for coded values as follows,
Antioxidant activity (%) = 53.82 − 0.22X1 −1.98X2 − 1.13X3 − 7.05X12 + 0.29X22 +6.45X32 + 1.01X1X2 − 1.69X1 X3 − 8.51X2X3
Extraction yield (%) = 30.40 − 6.25X1 +4.20X2 +3.35X3 − 4.70X12 +1.00X22 − 1.90X32 + 1.50X1X2 −0.20X1X3 + 0.50X2X3
Among extraction variables, quadratic term (X12) of MeOH concentration showed the most significant effect on antioxidant activity with p-value of <0.001 (Table 2). The linear (X3) and quadratic term (X32) of solvent/sample ratio together with the interaction term of extraction temperature with p values of 0.005, 0.007 and 0.002, respectively. Other variables, however, did not show any significant effect on antioxidant activity of lotus leaves in our present study.
Three dimensional response surface plots are consistent with multiple regression analysis. Extraction solvent showed dramatic effect on antioxidant activity of lotus leaves. Antioxidant activity of lotus leaves was increased with increasing MeOH concentration but decreased thereafter, which showed the importance of quadratic term (X12) of MeOH concentration (Figs. 2A and 2B). Similar quadratic pattern of solvent/sample ratio was observed at fixed extraction temperature (Fig. 2B).
Based on our results, an optimization for extraction condition of lotus leaves for maximum antioxidant activity was suggested by RSM and verified by experiment. Optimal extraction condition for maximum antioxidant activity was determined as MeOH concentration in water, 50.0%, temperature, 10.0℃, and solvent/sample ratio, 40, which predicted 70.3% antioxidant activity (Table 4). Extraction of lotus leaves extract prepared under optimized condition showed 70.1% antioxidant activity, which was well matched with predicted values.
Phenolic compounds contribute the antioxidant activity of lotus leaves. Therefore, correlation between antioxidant activity and phenolic content was investigated. The total phenolic content in lotus leaves extract was varied from 67.3 to 140.4 µg GAE/mg extract depending on extraction conditions (Table 1) and antioxidant activity was quite proportional to phenolic content with R2 value of 0.610 (Fig. 3). These results suggested that antioxidant activity of lotus leaves was achieved by phenolic constituents to some extent, which is also supported by the antioxidant activity of its constituents of quercetin (3) and trans-N-feruloyltyramine (4) (Fig. 1).
Although lotus leaves are widely used for food or tea, other parts of the lotus such as the fruit, flowers, seeds and roots are available and have been used in traditional medicine for a long time. Different parts of plant may contain different types of constituents which contribute to the biological activities.2427 In other aspect, plants use similar biosynthetic pathway among different parts, therefore, they share common structure or substituents which are characteristic to each plant.2526 Therefore, the antioxidant activity of different parts of lotus such as flowers, seeds and roots were also compared.
As shown in Table 5, the flower showed the most potent antioxidant activity followed by leaves, seeds and roots. Due to the importance of phenolic contents for antioxidant activity of lotus, the total phenolic content of each part was measured. The total phenolic content was the highest in flower, followed by leaves, seeds and roots, which showed the correlation with antioxidant activity. These results show that different parts of lotus commonly contains phenolic constituents, however, the amounts and structures are different among different parts. Therefore, other parts also can be used as important antioxidant resources for further development.
In conclusion, lotus leaves are widely used for tea and also consumed as healthy foods for its diverse activity. Therefore, extraction condition was optimized using response surface methodology for maximal efficacy. In addition, constituents and antioxidant activity of other parts of the lotus such as the flowers, seeds and roots have been compared. Our present study presented optimized extraction condition of lotus leaves for maximal antioxidant activity. Among parts of louts, flower showed the most potent antioxidant activity with high phenolic contents. Therefore, our present study will provide efficient extraction condition of lotus leaves for maximal activity and suggested other parts as important antioxidant resources for further development.
Figures and Tables
 | Fig. 2Response surface plots of antioxidant activity by MeOH concentration (X1), extraction temperature (X2) and solvent/sample ratio (X3). |
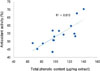 | Fig. 3Correlation between antioxidant activity and phenolic content of the lotus leaves extract from different extraction condition. |
Table 2
Regression coefficients and their significances in the second-order polynomial regression equation

Acknowledgement
This work was supported by Basic Science Research Program (2018R1D1A1A09082316) through the National Research Foundation of Korea.
References
1. Zhu MZ, Wu W, Jiao LL, Yang PF, Guo MQ. Molecules. 2005; 20:10553–10565.
4. Du H, You JS, Zhao X, Park JY, Kim SH, Chang KJ. J Biomed Sci. 2010; 17:S1–S42.
6. Nakamura S, Kasahima S, Tanabe G, Oda Y, Yokota N, Fujimoto K, Matsumoto T, Sakuma R, Ohta T, Ogawa K, Nishida S, Miki H, Matsuda H, Muraoka O, Yoshikawa M. Bioorg Med Chem. 2013; 21:779–787.

7. Ahn JH, Kim ES, Lee C, Kim S, Cho SH, Hwang BY, Lee MK. Bioorg Med Chem Lett. 2013; 23:3604–3608.

8. Paudel KR, Panth N. Evid Based Complement Alternat Med. 2015; 2015:789124.
10. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Free Radic Biol Med. 2010; 49:1603–1616.
13. Alasalvar C, Bolling BW. Br J Nutr. 2015; 113:S68–S68.
15. Park YS, Towantakawanit K, Kowalska T, Jung ST, Ham KS, Heo BG, Cho JY, Yun JG, Kim HJ, Gorinstein S. J Med Food. 2009; 12:1057–1064.

21. Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EG, Portugal LA, dos Reis PS, Souza AS, dos Santos WN. Anal Chim Acta. 2007; 597:179–186.

27. Chung IM, Lim JJ, Ahn MS, Jeong HN, An TJ, Kim SH. J Ginseng Res. 2016; 40:68–75.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download