Abstract
Microbial wound infection prolonged the hospitalization and increase the cost for wound management. Silver is commonly used as antimicrobial wound dressing. However, it causes several adverse side effects. Hence, this study was aimed to evaluate the antimicrobial efficiency of Swietenia macrophylla seed extract on clinical wound pathogens. Besides, the bioactive constituents of the seed extract were also determined. S. macrophylla seeds were extracted with methanol by maceration method. The seed extract inhibited 5 test bacteria and 1 yeast on disc diffusion assay. The antibacterial activity was broad spectrum, as the extract inhibited both Gram positive and Gram negative bacteria. On kill curve analysis, the antibacterial activity of the seed extract was concentration-dependent, the increase of extract concentration resulted in more reduction of bacterial growth. The extract also caused 99.9% growth reduction of Bacillus subtilis relative to control. A total of 21 compounds were detected in gas chromatography- mass spectrometry analysis. The predominant compounds present in the extract were oleic acid (18.56%) and linoleic acid (17.72%). In conclusion, the methanolic extract of S. macrophylla seeds exhibited significant antimicrobial activity on clinical wound pathogens. Further investigations should be conducted to purify other bioactive compounds from the seeds of S. macrophylla.
Go to : 
REFERENCES
(1). Bowler P. G., Duerden B. I., Armstrong D. G.Clin. Microbiol. Rev. 2001; 14:244–269.
(2). Abeysinghe P. D., Weeraddana C. S. J.Pharm. Biomed. Sci. 2011; 11:1–4.
(3). Rai M. K., Deshmukh S. D., Ingle A. P., Gade A. K. J.Appl. Microbiol. 2012; 112:841–852.
(4). Panyala N. R., Pena-Mendez E. M., Havel J. J.Appl. Biomed. 2008; 6:117–129.
(5). Gillies A. C. M., Navarro C., Lowe A. J., Newton A. C., Hernández M., Wilson J., Cornelius J. P.Heredity. 1999; 83:722–732.
(6). Eid A. M. M., Elmarzugi N. A., El-Enshasy H. A.Int. J. Pharm. Pharm. Sci. 2013; 5:47–53.
(7). Paritala V., Chiruvella K. K., Thammineni C., Ghanta, R. G.;Mohammed A. Braz. J.Pharm. 2015; 25:61–83.
(8). Pamplona S., Sá P., Lopes D., Costa E., Yamada E., e Silva C., Arruda M., Souza J., da Silva M.Molecules. 2015; 20:18777–18788.
(9). Tong W. Y., Ang S. N., Darah I., Latiffah Z.World J. Pharm. Pharm. Sci. 2014; 3:121–132.
(10). Chen Q., Fung K. Y., Lau Y. T., Ng K. M, Lau D. T. W.Food Bioprod. Process. 2016; 98:236–243.
(11). Onivogui G., Letsididi R., Diaby M., Wang L, Song Y.Asian Pac. J. Trop. Biomed. 2016; 6:20–25.
(12). Siripatrawan U., Kaewklin P.Food Hydrocolloid. 2018; 84:125–134.
(13). Panghal M., Singh K., Kadyan S., Chaudary U., Yadav J. P.Burns. 2015; 41:812–819.
(14). Habermann E., Pereira V. D. C., Imatomi M., Pontes F. C., Gualtieri S. C. J. Braz. J.Bot. 2017; 40:33–40.
(15). Ertas A., Boga M., Gazioglu I., Yesil Y., Hasimi N., Ozaslan C., Yilmaz H., Kaplan M.Chiang Mai J. Sci. 2016; 43:89–99.
(16). Pushparaj S. P., Nellore J., Balaraman R. M., Sekar U., Tippabathani J.Artif. Cells Nanomed. Biotechnol. 2018; 46:268–273.
(17). Adesanwo J. K., Ogundele S. B., Akinpelu D. A., McDonald A. G. J.Explor. Res. Pharmacol. 2017; 2:67–77.
Go to : 
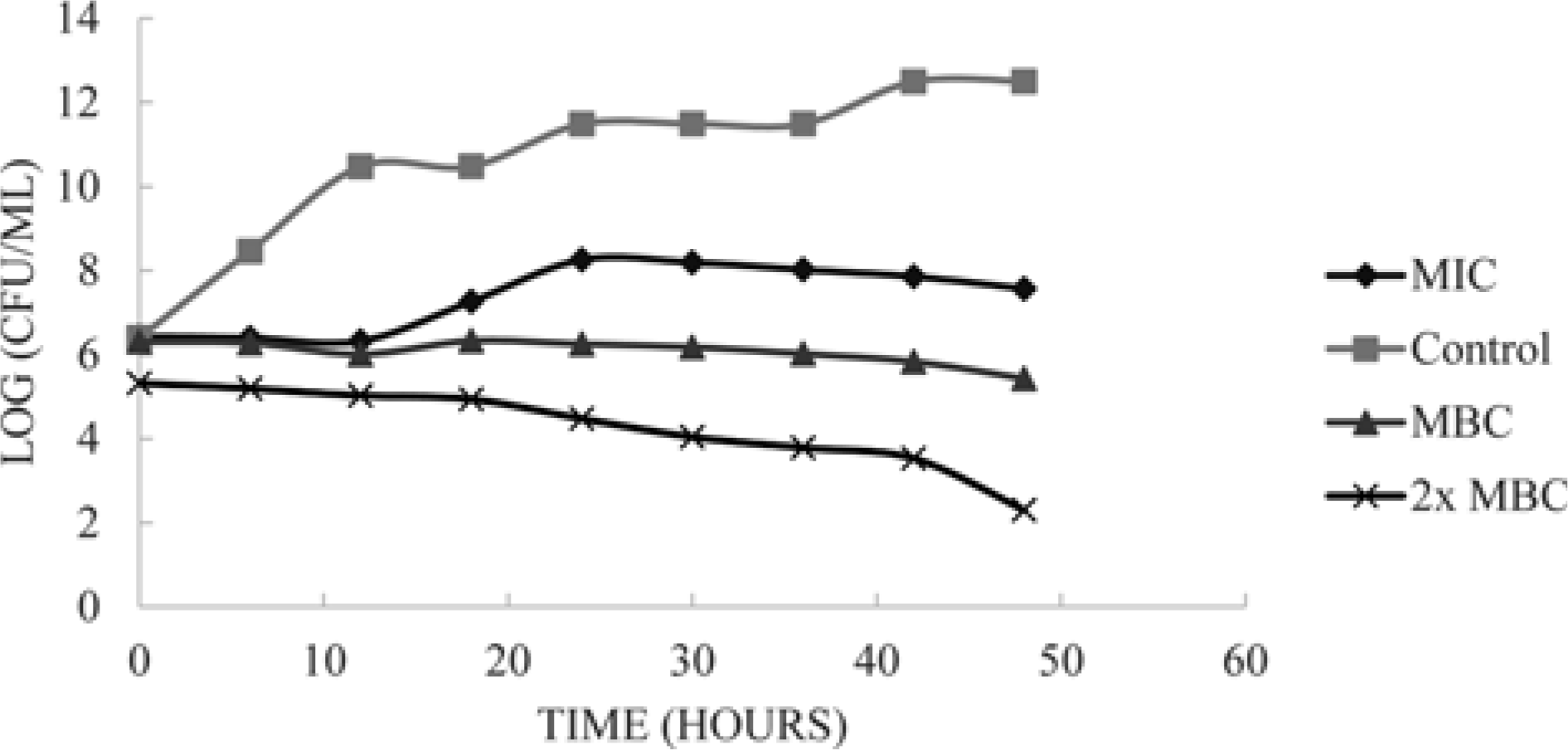 | Fig. 1.The kill curves of B. subtilis when exposed to different concentrations of S. macrophylla seed extracts. The antibacterial activity of the extract was concentration-dependent. |
Table 1.
Antimicrobial activity of S. macrophylla seed extract on wound microorganisms.
Table 2.
The MIC and MBC of S. macrophylla seed extract on various clinical wound pathogens recorded on broth microdilution assay.
| Tested microorganisms | MIC (mg/ml) | MBC (mg/ml) |
|---|---|---|
| B. cereus | 3.13 | 6.25 |
| S. aureus | 6.25 | 25.00 |
| B. subtilis | 1.56 | 3.13 |
| S. boydii | 12.50 | 25.00 |
| A. anitratus | 12.50 | 25.00 |
| C. utilis | 12.50 | 25.00 |
Table 3.
The GC/MS analysis showed the presence of 21 compounds in the Fraction 1 from methanolic extract of S. macrophylla seeds.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download