Abstract
Phytochemical studies were performed to identify the active principles of Phryma leptostachya var. asiatica (Phyrymaceae) for anti-inflammation. The anti-inflammatory activity was assessed by measuring the inhibition rate on nitric oxide (NO) formation in lipopolysaccharide (LPS)-activated macrophage 264.7 cells. Of the five compounds including ursolic acid, phrymarolin I, harpagide, haedoxancoside A, and acteoside isolated from this plant, ursolic acid showed the most prominent inhibition of NO formation. Therefore, ursolic acid may be the anti-inflammatory principle of Phryma leptostachya var. asiatica.
Phrima leptostachya var. asiatica (Phrymaceae) is a perennial herb growing in the shading place. This plant is distributed in Korea, Japan, and China as well as Siberia and North-East America. Paripul,the Korean name of this plant, was named because its root has been used to kill flies. In addition, this plant has been also used to treat allergic dermatitis and itching and to prevent cancer disease.1
The constituents of the lignans of phrymarolin I and II,23 V and B45 possessing the basic structure of 1,2-dioxygenated-3,7-dioxabicyclo[3.3.0]octane, haedoxancoside A belonging to sesquilignan,5 and ursolic acid as the triterpene acid6 were previously reported. Furthermore, the larvicidal activity ofleptostachyol acetate, a lignan of P. leptostachya var. asiatica, has been also reported.78
Nitric oxide (NO) is a simple and gaseous mediator produced by nitric oxide synthase (NOS) including inducible nitric oxide synthase (iNOS). NO is involved in pathophysiological conditions such as inflammatory- and autoimmune diseases.9 The expression of iNOS is induced by pro-inflammatory cytokines and bacterial lipopolysaccharide (LPS).10 Therefore, anti-inflammatory effect is assessed by measuring the amount of NO in lipopolysaccharide (LPS)-induced murine macrophage RAW 264.7 cells.
Jung et al.1 reported that the root extract of P. leptostachya var. asiatica has anti-inflammatory effect via the mechanism of anti-oxidative and the inhibition of iNOS and cyclooxygenase-2 activities in LPS-activated macrophage cells. However, the active principle of P. leptostachya var. asiaticafor anti-inflammation has not been reported yet. Therefore, in the present study, we aimed to identify which compounds are mainly responsible for anti-inflammatory activity of this plant.
The whole plant of Phyryma leptostachya var. asiatica Hara (Phrymaceae), was collected from the mountain area in Wonju city, Gangwon-do, Korea. The plant was washed, dried, and cut for extraction. The plant was identified by Prof. Byung-Min Song, Department of Forest Science, Sangji Univerisity, Korea. A voucher specimen (natchem-#87) was deposited in the Laboratory of Natural Products Chemistry, Sangji University, Korea.
The plant material (892 g) was extracted with MeOH (each, 5.0 L) three times under reflux. The extracted solution was filtered and concentrated under reduced pressure on a rotatory evaporator. The viscous MeOH extract was further subjected to freeze-drying to give a solid MeOH extract (123.0 g, extraction yield 13.3%).
The MeOH extract was suspended in H2O (2.0 L), and partitioned with hexane (2.0 L) three times. The hexane fraction was further concentrated in vacuo to give a CHCl3 fraction (24.0 g). In the same method, the residual MeOH extract was successively fractionated with CHCl3, EtOAc, and BuOH, respectively, to give a CHCl3 fraction (18.5 g), EtOAc fraction (4.55 g), and BuOH fraction (28.0 g).
After that solvent fractionation, the hexane fraction was further fractionated by the column chromatography. The hexane fraction was developed over diaion HP-20 column chromatography with 1.0 L MeOH, and then eluted with 1.0 L MeOH-CHCl3 (1:1). The MeOH-CHCl3 (1:1)-eluted solution was concentrated in vacuo to yield a MeOH-CHCl3 fraction.
In addition, the BuOH fraction was further subjected to diaion HP-20 column chromatography to remove sugars and ionic substances. The BuOH fraction was washed with H2O (2.0 L) over the column, and then eluted with MeOH (2.0 L). The MeOH solution was concentrated to yield 8.29 g MeOH fraction.
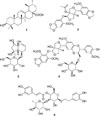
The MeOH-CHCl3 fraction obtained from the hexane fraction was subjected to silica gel column (40 µm, 165 g, 48 × 170 mm, Hi-Flash column, Yamazen Co., Japan) chromatography using CHCl3-MeOH-H2O (8:1:1, lower phase) and collected by each 50 mL. After checking TLC, the fractions #25–28 and #36–39 were concentrated to afford PLA-#25–28 and PLA-#36–39, respectively. PLA-#25–28 was washed with MeOH to yield compound 1. PLA-#36–39 was recrystallized from MeOH to yield compound 2. Compounds 1 and 2 were identified as ursolic acid (Lee et al., 2002) and phrymarolin I (Taniguchi and Oshima, 1972), respectively, by comparisons of 1H- and 13C-NMR spectroscopic data with literatures.
White powder, UV λmax MeOH (log ε): 220 (2.83) nm; IR νmax (KBr) cm−1: 3400 (broad, OH), 1090(COOH); 1H-NMR (600MHz, pyridine-d5) and 13C-NMR (150 MHz, pyridine-d5) δ: Literature.6
Amorphous powder, IR νmax (KBr) cm−1: 3008 (broad, OH), 1733 (ester), 1634, 1502 (aromatic C=C); 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) δ: Literature.2
The MeOH fraction obtained by eluting the BuOH fraction over diaion HP-20 column with MeOH was subjected to silica gel column chromatography (40 µm, 165 g, 48 × 170 mm, Hi-Flash column, Yamazen Co., Japan) with the solvent of CHCl3-MeOH-H2O (65:35:10, lower phase), and collected by each 50 mL. After checking TLC, the fractions #39–44, #50–58, and #72–88 were concentrated, respectively, and precipitated from MeOH to yield compounds 3, 4, and 5. The three compounds 3, 4, and 5 were identified as harpagide,11 haedoxancoside A,5 and acteoside12 by comparisons of spectroscopic data with literatures.
Amorphous powder frm MeOH, mp 228–229, IR νmax (KBr) cm−1: 3358, 1643, 1250, 1047; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) δ: Literature.11
White powder, mp 181 – 183 ℃, UV λmax (MeOH) nm (log ε) : 293 (4.05), 234 (4.08); IR νmax (KBr) cm−1: 34783006, 2941, 1600, 1500; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150MHz, CD3OD) δ: Literature.5
UV λmax (MeOH) nm (log ε) : 328 (4.11); IR νmax (KBr) cm−1: 3403 (broad, O-H), 1698 (C=O), 1631 (olefinic C=C), 1100 – 1000 (glycosidic C-O); 1H-NMR (600MHz, CD3OD) δ: Literature.12
Murine macrophage RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA), and cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics-antimycotics (PSF; 100 units/ml penicillin G sodium, 100 ng/mL streptomycin, and 250 ng/mL amphotericin B). The cells were incubated at 37 ℃ under a humidified atmosphere containing 5% CO2.
After the supernatant was collected for iNOS assay, MTT solution (final concentration of 500 µg/mL) was added to each well and incubated for 4 h at 37 ℃. The culture media was aspirated, and dimethyl sulfoxide (DMSO) was added to dissolve the dye. The absorbance was measured at 570 nm using VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA), and the percent survival was determined by comparison with a control group (LPS+).
Murine RAW 264.7 cells were seeded in 24-well plates (2 × 105 cells/mL). The next day, culture media were changed to 1% FBS-DMEM with sample treatment. After 1 h, LPS (1 µg/mL) was added, except LPS- control, to stimulate NO production. The amount of NO production in culture media was determined by Griess reaction after 18 h incubation. Briefly, 100 µL of culture media was collected per each well and 180 µL of Griess reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in H2O and 1% sulfanilamide in 5% H3PO4) was added. The absorbance was measured at 540 nm. The nitrate concentration was determined by comparison with sodium nitrite standard curve. Percent inhibition was calculated using following formula: [1 − (NO level of test samples/NO levels of vehicle-treated control)] × 100. The IC50 value was calculated through non-linear regression analysis using TableCurve 2D v5.01 (Systat Software Inc., San Jose, CA, USA)
The iNOS is an enzyme responsible for the production of nitric oxide (NO). Recently, anti-inflammatory activity is frequently assayed by measuring the iNOS inhibition activity.13 In the present study, iNOS assay was performed to identify the anti-inflammatory compounds since the active compounds from P. leptostachya var. asiaticahave not been reported.
In this study, the iNOS inhibition activity was determined by measuring the amount of nitrate in LPS-activated macrophage 264.7 cells. Cells were pre-treated with 40 µg/ml of each fractions for 1 h and LPS (1 µg/mL) were added to stimulate the cells to produce NO. The iNOS inhibition rate (%) of the MeOH extract and its fractions were shown in Table 1, and cell viability was also tested in the same condition. The MeOH extract significantly reduced the formation of NO by 41.0%. The hexane fraction effectively reduced the NO formation by 63.8% which was more effective than other three fractions. While the MeOH, hexane, and EtOAc fractionsdid not affect the cell viability, the CHCl3 fraction was relatively cytotoxic with 26.4% cell viability at 40 µg/mL.
Therefore, the hexane fraction was chromatographed to isolate the active substance. The two substances of ursolic acid6 and phrymarolin I2 were identified by comparison of 1H-NMR and 13C-NMR spectroscopic data with literatures, as shown in Fig. 1. Phrymarolin I possessing the basic skeleton of 1,2-dioxygenated-3,7-dioxabicyclo[3.3.0]octane is known to have a larvicidal activity.78 The three compounds 3, 4, and 5 isolated from the BuOH fraction were identified as harpagide,11 haedoxancoside A,5 acteoside12 by 1H-NMR and 13C-NMR spectra. Of the five compounds isolated, harpagide and acteoside has not been reported from P. leptostachya var. asiatica.
The inhibition rate of the four compounds (ursolic acid, phrymarolin I, haedoxancoside A and acteoside) are shown in Table 2. The AMT (2-Amino-5,6-dihydro-6-methyl-4H-1,3-thiazine), a known iNOS inhibitor, was used as a positive control. Among the four compounds, ursolic acid showed the highest inhibition rate (80.6%), and other compounds showed inhibition rate lower than 50%. Therefore, the inhibition of NO formation and cell viability of ursolic acid were further tested at 10, 20, and 40 µg/mL, respectively (Fig. 2). The IC50 value was determined to be 20.8 µg/mL,and the inhibition of NO production by ursolic acid was not derived from the cell viability.
Although the anti-inflammatory and inhibitory effects of NO formation by ursolic acid have been reported,1415 the inhibitory activities of phrymarolin I and haedoxancoside A on NO formation are newly discovered in the present study. In conclusion, ursolic acid exhibited the most potent anti-inflammatory activity without cytotoxicity. In addition, harpagide and acteoside were first isolated from P. leptostachia var. asiatica.
Figures and Tables
Fig. 2
Effect of compound 1 on NO formation in LPS-activated macrophage 264.7 cells. Cells were pretreated with different concentrations of ursolic acid and stimulated with LPS (1 µg/mL). The amount of nitrate was measured by Griess reaction. The cell viability was measured using MTT. The data are presented as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.005 by t-test.
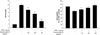
References
1. Jung HJ, Cho YW, Lim HW, Choi H, Ji DJ, Lim CJ. Biomol Ther. 2013; 21:72–78.
2. Tanigichi E, Oshima Y. Agric Biol Chem. 1972; 36:1013–1025.
3. Taniguchi E, Oshima Y. Agric Biol Chem. 1972; 36:1489–1496.
4. Xiao X, Ji Z, Zhang J, Shi B, Wei S, Wu W. Chem Nat Comp. 2013; 49:21–23.
5. Chen C, Zhu H, Zhao D, Deng J, Zhang Y. Helv Chim Acta. 2013; 96:1392–1396.
6. Lee S, Min B, Kho Y. Arch Pharm Res. 2002; 25:652–654.
7. Seo SM, Park IK. Parasitol Res. 2012; 110:1849–1853.
11. Manguro LOA, Lemmen P, Hao P. Rec Nat Prod. 2011; 5:147–157.
12. Schlauer J, Budzianowski J, Kukulczanka K, Ratajczak L. Acta Soc Bot Pol. 2004; 73:9–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download