Abstract
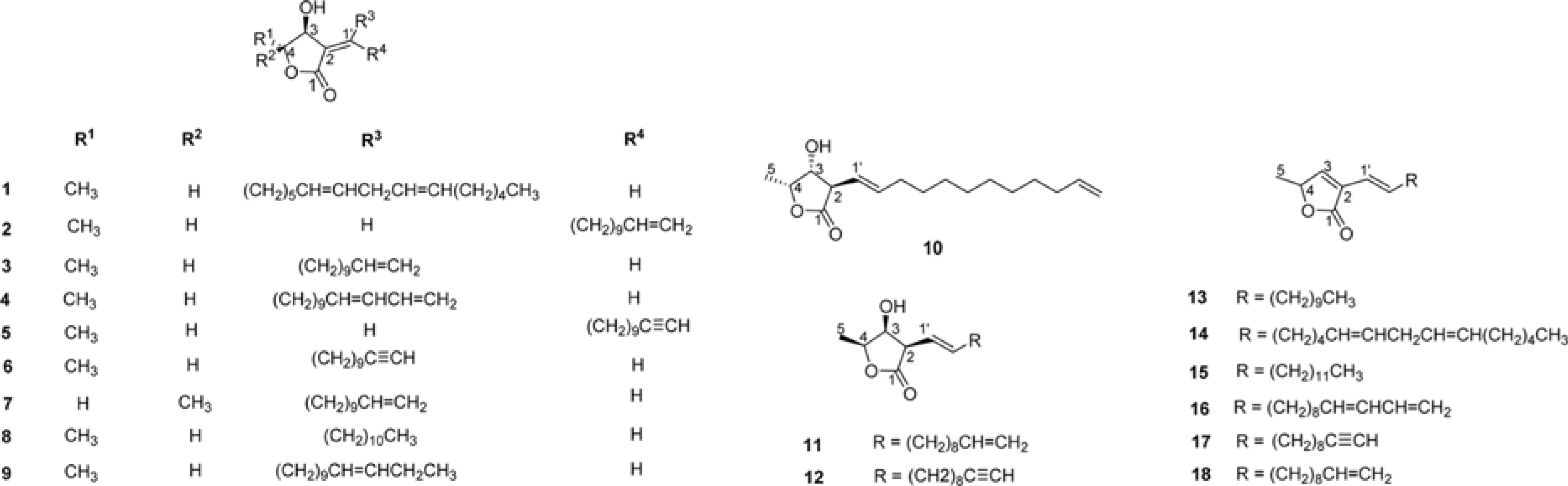
From the pericarps of Litsea japonica (Thunb.) Jussieu, eighteen butanolide derivatives (1–18) were evaluated for their cytotoxic activity against HeLa, HL-60, and MCF-7 cells. Compounds 1–9 with 2-alkylidene-3-hydroxy-4-methylbutanolides structure exhibited cytotoxic activities against cancer-cell lines. Among them, compound 8 (litsenolide D2) exhibited the most potent cytotoxicity against the tested cell lines, including HeLa, HL-60, and MCF-7, with IC50 values of 17.6 ± 1.3, 4.2 ± 0.2, and 12.8 ± 0.0 µM, respectively. Compound 8 induced apoptosis in a dose-dependent manner. Annexin V/Propidium Iodide (PI) double staining confirmed that 8 effectively induced apoptosis in MCF-7 cells. To the best of our knowledge, we have reported cytotoxic activity of butanolides from L. japonica against these cancer-cell lines for the first time.
Go to : 
REFERENCES
(1). Wang Y. S., Wen Z. Q., Li B. T., Zhang H. B., Yang J. H. J.Ethnopharmacol. 2016; 181:66–107.
(2). Lee S. S., Lin Y. J., Chen C. K., Liu K. C. S., Chen C. H. J.Nat. Prod. 1993; 56:1971–1976.
(3). Dong S., Tong X., Li J., Huang C., Hu C., Jiao H., Gu Y.Neural. Regen. Res. 2013; 8:3193–3202.
(4). Yang Y., Jiang J., Qimei L., Yan X., Zhao J., Yuan H., Qin Z., Wang M.Molecules. 2010; 15:7075–7082.
(5). Wang L., Zhao J. F., Zeng X. H., Xie M. J., Yang X. D., Zhang H. B., Li L. J.Asian Nat. Prod. Res. 2009; 11:1028–1031.
(6). Tsai I. L., Jeng Y. F., Duh C. Y., Chen I. S. J.Chin. Pharm. Sci. 2001; 53:291–301.
(7). Min B. S., Lee S. Y., Kim J. H., Kwon O. K., Park B. Y., An R. B., Lee J. K., Moon H. I., Kim T. J., Kim Y. H., Joung H., Lee H. K. J.Nat. Prod. 2003; 66:1388–1390.
(8). Chen I. S., Lai-Yaun I. L., Duh C. Y., Tsai I. L.Phytochemistry. 1998; 49:745–750.
(9). Cheng H. I., Lin W. Y., Duh C. Y., Lee K. H., Tsai I. L., Chen I. S. J.Nat. Prod. 2001; 64:1502–1505.
(10). Tanaka H., Takaya Y., Toyoda J., Yasuda T., Sato M., Murata J., Murata H., Kaburagi K., Iida O., Sugimura K., Sakai E.Phytochem. Lett. 2015; 11:32–36.
(11). Ngo Q. T., Cao T. Q., Tran P. L., Kim J. A., Seo S. T., Kim J. C., Woo M. H., Lee J. H., Min B. S.Bioorg. Med. Chem. Lett. 2018; 28:2109–2115.
(12). Guon T. E., Chung H. S.Nat. Prod. Sci. 2017; 23:227–234.
(13). Taher M., Aminuddin A., Susanti D., Aminudin N. I., On S., Ahmad F., Hamidon H.Nat. Prod. Sci. 2016; 22:122–128.
(14). Takeda K., Sakurawi K., Ishii H.Tetrahedron. 1972; 28:3757–3766.
(15). Zhao Y., Guo Y. W., Zhang W.Helv. Chim. Acta. 2005; 88:349–353.
(16). Tanaka H., Nakamura T., Ichino K., Ito K., Tanaka T.Phytochemistry. 1990; 29:857–859.
(17). Ham Y. M., Ko Y. J., Song S. M., Kim J., Kim K. N., Yun J. H., Cho J. H., Ahn G., Yoon W. J. J.Funct. Foods. 2015; 13:80–88.
(18). Cao H. Q., Lee B. M., Jung Y. W., Nguyen V. T., Kim J. A., Min B. S.Nat. Prod. Commun. 2017; 12:259–260.
(19). Shin M., Lee B. M., Kim O., Tran H. N. K., Lee S., Hwangbo C., Min B. S., Lee J. H.Food Funct. 2018; 9:3895–3905.
(20). Bold R. J., Termuhlen P. M., McConkey D. J.Surg. Oncol. 1997; 6:133–142.
(21). Yang H. L., Chen C. S., Chang W. H., Lu F. J., Lai Y. C., Chen C. C., Hseu T. H., Kuo C. T., Hseu Y. C.Cancer Lett. 2006; 231:215–227.
(23). Park J. H., Noh T. H., Wang H., Kim N. D., Jung J. H.Nat. Prod. Sci. 2015; 21:282–288.
Go to : 
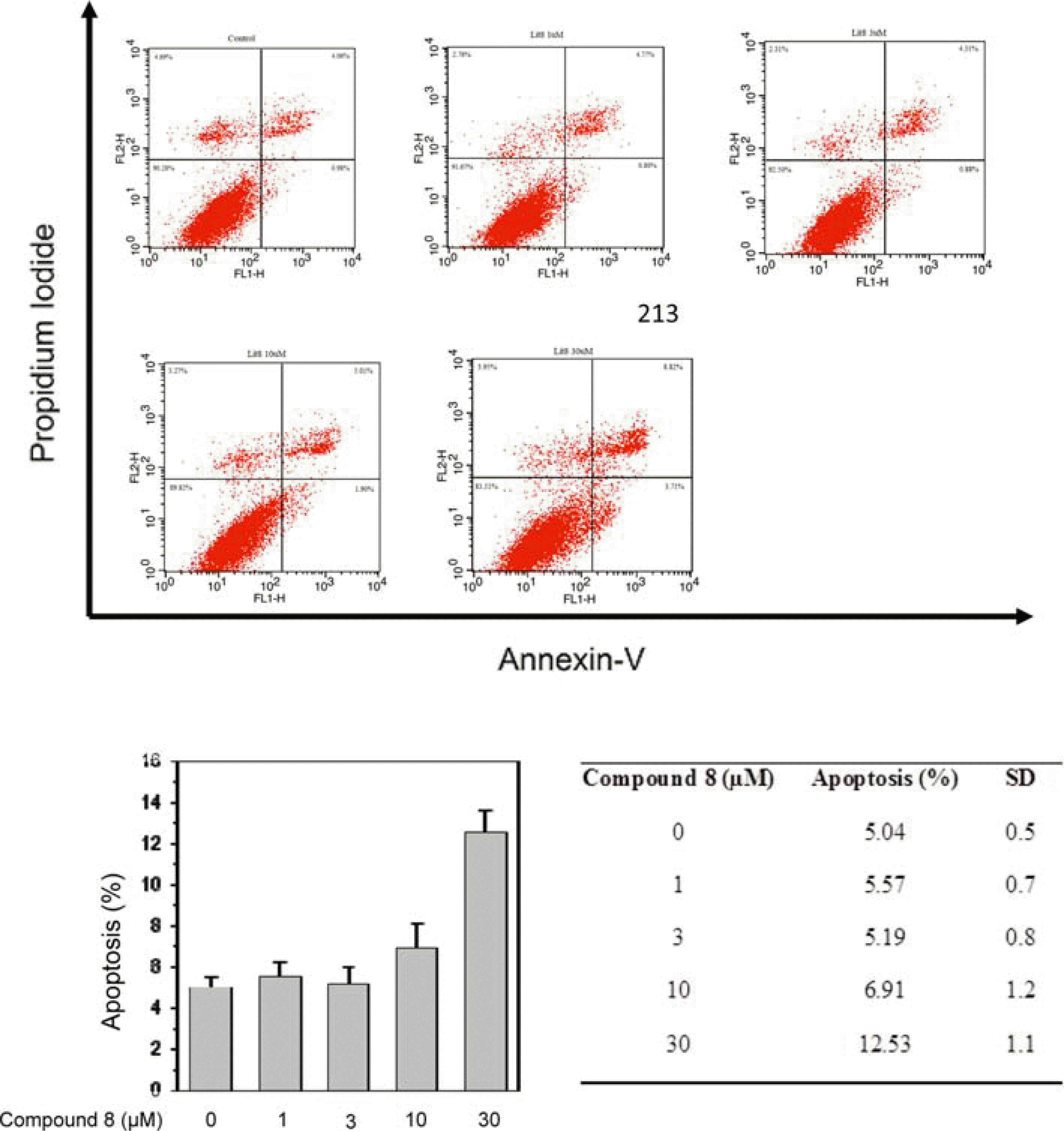 | Fig. 3.Flow cytometric analysis of compound 8-induced apoptosis in MCF-7 cell using annexin-V-FLUOS/PI. Cells (1 × 106 cells) were incubated with indicated concentration of 8 for 24 h and stained with annexin-V-FLUOS/PI to analysis apoptosis and necrotic cell populations. Cells in the lower right quadrant represented apoptosis and upper right quadrant. Data are representative of one of three similar experiments. |
Table 1.
Cytotoxic activities of compounds 1–18 against Hela, HL-60, and MCF-7 cell lines
| Compds | IC50, µ Ma | ||
|---|---|---|---|
| HeLa | HL-60 | MCF-7 | |
| 1 | 47.0 ± 0.2 | ± 0.4 | 38.7 ± 0.3 |
| 2 | 18.4 ± 0.8 | 5.5 ± 0.6 | 38.2 ± 0.5 |
| 3 | 45.2 ± 0.9 | 22.1 ± 1.8 | 72.2 ± 0.6 |
| 4 | 34.2 ± 0.4 | 14.0 ± 0.6 | 24.4 ± 0.7 |
| 5 | 80.3 ± 2.0 | 26.3 ± 0.0 | 67.5 ± 0.1 |
| 6 | 100 | 29.6 ± 0.8 | > 100 |
| 7 | 27.7 ± 0.2 | 11.2 ± 1.5 | 27.2 ± 0.2 |
| 8 | 17.6 ± 1.3 | 4.2 ± 0.2 | 12.8 ± 0.0 |
| 9 | 19.1 ± 1.1 | 8.1 ± 0.3 | 22.9 ± 1.1 |
| 10 | 100 | 23.2 ± 0.5 | > 100 |
| 11 | 100 | 54.9 ± 0.6 | > 100 |
| 12 | 54.5 ± 0.3 | 47.6 ± 0.7 | - |
| 13 | - | 30.2 ± 0.9 | > 100 |
| 14 | 100 | 37.0 ± 1.2 | > 100 |
| 15 | 95.4 ± 0.3 | 28.3 ± 1.6 | > 100 |
| 16 | - | - | - |
| 17 | - | 27.4 ± 1.3 | > 100 |
| 18 | - | 32.6 ± 0.9 | - |
| Adriamycinb | 0.67 ± 0.5 | 0.24 ± 0.06 | 53.4 ± 0.2 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download