This article has been
cited by other articles in ScienceCentral.
A 63-year-old woman presented with exertional dyspnea. She had received 6 cycles of adriamycin-based chemotherapy 5 years prior and an additional 6 cycles of the same regimen due to cancer metastasis. Treatments were completed 3 weeks before onset of dyspnea. The cumulative dose of adriamycin was 563 mg/m
2. Her B-type natriuretic peptide level was 4,116 pg/mL (reference: < 100 pg/mL), and transthoracic echocardiography revealed severe dysfunction [left ventricular ejection fraction (LVEF) 33%, global longitudinal strain (GLS) −8.3%]. We started heart failure medications, and her symptoms improved. Follow-up echocardiography showed gradual improvement: LVEF changed from 33% to 52% to 61% (
Movie 1,
2,
3) and GLS from −8.3% to −13.0% to −17.1% (
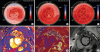
Figures 1A-C). Subsequently, beta-blocker dose was halved due to low blood pressure and no further complaints of dyspnea. One month later, however, she presented at an emergency department with resting dyspnea with decreased LVEF of 33%. After the stabilization period, we performed echocardiography; surprisingly, the LVEF again recovered to 58.6% and GLS to −17.5%. Cardiac magnetic resonance imaging (3T, Verio, Siemens, Enlangen, Germany) showed elevated native T1 value (1,394–1,411 ms, reference: 1,278 ± 30 ms), T2 value (55–59 ms, reference: 40.5 ± 2.5 ms), and extracellular volume fraction (ECV, 32.5-33.3%, reference: 27.4 ± 2.4%), although we did not detect late gadolinium enhancement (
Figures 1D-F). These findings suggest diffuse interstitial fibrosis combined with edema and inflammation, which might explain the myocardial vulnerability. Consistent with recent studies,
1)2)3)4) our case shows the usefulness of multi-modality imaging for evaluation of myocardial vulnerability.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download