Abstract
Background and Purpose
The "closing-in" phenomenon refers to the tendency to copy near or overlap a model while performing figure-copying tasks. The mechanisms underlying the closing-in phenomenon have not been fully elucidated, and previous studies only investigated the mechanisms through neuropsychological tests. We investigated the neuroanatomical correlates of the closing-in phenomenon using voxel-based morphometry (VBM).
Methods
Thirty-eight patients diagnosed with probable Alzheimer's disease (AD) and 21 normal controls were included. All subjects underwent neuropsychological testing to diagnose dementia and magnetization prepared rapid acquisition gradient echo brain magnetic resonance imaging for the voxel-based statistical analysis. The subjects were asked to copy the modified Luria's alternating squares and triangles to quantify the closing-in phenomenon. We applied SPM8 for the VBM analysis to detect gray matter loss associated with the closing-in phenomenon.
Results
The patients with probable AD showed a higher closing-in score than that of the normal control subjects (p<0.0001). The VBM analysis revealed more parietal and temporal atrophy in the patients with AD than that in the normal control group. Moreover, atrophy of the orbito-frontal area was associated with the closing-in phenomenon.
Conclusions
The closing-in phenomenon is dysfunction while performing figure-copying tasks and is more common in patients with AD. The analysis of the orbito-frontal area, which is associated with inhibiting primitive reflexes, revealed that the closing-in phenomenon is an imitation behavior commonly observed in patients with frontal lobe damage.
The "closing-in" phenomenon is defined as a tendency to draw objects as closely as possible or overlap the figures in severe cases.1 It was first described by Mayer-Gross.2 Since then, this phenomenon has been reported in patients with various brain lesions and in normal children.3,4,5 It frequently occurs in patients with Alzheimer's disease (AD) and is considered a specific neurocognitive indicator of AD.1,6
Little is known about the mechanisms by which the closing-in phenomenon occurs. However, several hypotheses have been proposed to explain the pathophysiology of the closing-in phenomenon. Mayer-Gross2 described the phenomenon as an aspect of constructional apraxia that reflects "the fear of empty space". In addition, de Ajuriaguerra et al.4 described the closing-in phenomenon as rigid and fixed responses, such as a magnet with an attraction. These authors noted that it is similar to primitive behavioral reflexes, such as grasping, sucking, and echolalia. In addition, McIntosh et al.7 defined "default behavior" as the closing-in phenomenon in which patients have an increased tendency to draw an object as closely as possible based on visual concentration. That is, these authors considered the closing-in phenomenon a primitive behavior. They also noted that it may occur as a result of frontal lobe dysfunction and may also arise from a lack of frontal executive function.
However, Kwak8 and Lee et al.9 considered the closing-in phenomenon as a compensatory behavior to overcome basic visuo-spatial impairment when patients visually analyze an object and then draw the objects as closely as possible to each other while concentrating on the objects. From this point of view, the closing-in phenomenon has been defined as a tendency to draw objects by shortening the distance between them to minimize impairment of the visuo-spatial analysis or working memory. In addition, it has also been regarded as the mechanism for visuo-spatial compensation.
Two hypotheses have been proposed to explain the closing-in phenomenon. The attraction hypothesis proposes that the closing-in phenomenon occurs due to primitive behavior and the compensation hypothesis proposes that it occurs when visuo-spatial functions are operated to compensate for impaired working memory. These hypotheses have indirectly been verified based on neurocognitive test results from patients showing the closing-in phenomenon. However, no objective imaging studies have been conducted to examine the mechanisms.
Thus, we used objectively measured and quantified the closing-in phenomenon. Then, we confirmed the regional gray matter atrophy associated with the phenomenon using a voxel-based morphometry (VBM) analysis. Thus, we attempted to identify the brain region associated with the closing-in phenomenon.9,10
We enrolled 38 patients with AD and 21 normal healthy controls who visited the Dementia Clinic at Seoul St. Mary's Hospital of the Catholic University of Korea during January 2009-December 2010. We recruited only patients with AD who were ≥65 years to rule out early onset AD. Of the patients who met the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria for the probable AD diagnosis,11 we recruited only those with mild-to-moderate AD whose Clinical Dementia Rating Scale (CDR) scores were 0.5 or 1.0.12 All subjects underwent the Mini-Mental State Examination (MMSE), the Korean Dementia Screening Questionnaire, the Geriatric Depression Scale (GDS), the Seoul Neuropsychological Screening Battery (SNSB)13 and brain magnetic resonance imaging (MRI) (T2, T1, fluid attenuated inversion recovery, and three-dimensional spoiled gradient echo images).
Exclusion criteria for the patient group were: 1) history of disease causing cognitive impairment identified on a clinical laboratory test (thyroid dysfunction, vitamin B12 or folic acid deficiency, syphilis, or metabolic encephalopathy), 2) psychiatric diseases that could cause cognitive impairment (any Diagnostic and Statistical Manual of Mental Disorders-IV axis disorders, including schizophrenia or bipolar disorder), 3) history of mental retardation, encephalitis, severe head trauma causing sustained (>1 hr) unconsciousness, brain tumor/post-traumatic hemorrhage, or subarachnoid hemorrhage, 4) physical and neurological deficits that could affect cognitive function test results such as loss of visual acuity, hearing difficulty, severe aphasic disorder, cancer with no remission, malignant tumor, severe hepatic disease, severe renal disease requiring kidney dialysis, acute, severe or unstable asthma, current diagnosis of uncontrolled peptic ulcer, recent 3-month history of gastrointestinal bleeding, non-insulin dependent diabetes, or sick sinus syndrome, 5) lesions on brain MRI scans suspected of causing cognitive impairment or findings suggestive of vascular dementia or post-traumatic brain injury, and 6) severe abnormalities of the white matter, as assessed by Fezekas visual rating scale.14
Exclusion criteria for the control group (normal healthy controls) were: 1) diseases impairing cognitive functions according to Christensen et al.,15 including Parkinson's disease, multiple sclerosis, cerebral palsy, Huntington's disease, encephalitis, epilepsy, cerebral infarction, history of brain injury, drug addiction, alcohol abuse, drug abuse, sustained (>1 hr) unconsciousness, or major psychiatric disorder, 2) history of neurological or psychiatric disease, 3) clinically notable findings on routine laboratory testing or brain MRI scans, and 4) lack of cognitive function.
The patients with AD and the normal healthy controls were asked to copy the modified Luria's alternating squares and triangles. Quantification of the closing-in phenomenon was based on the methods of Chin et al.10 and Luria.16
As shown in Fig. 1, the object was composed of nine figures (five quadrangles and four triangles), each of which was drawn alternately. Two triangles and two quadrangles were drawn sequentially, except in two regions. Each figure had a size of 10×10 mm. The overall length was 130 mm and the thickness of the line was 1 mm. The test sheet was printed in black on white A4-sized (297×210 mm) paper. The longer side was placed horizontally. In addition, the left upper point of the object was placed 70 mm from the upper end and 20 mm from the left end. The starting point was set at 50 mm inferior from the left end of the object, where the subjects were asked to begin to copy the figure. Then, scores for the closing-in phenomenon were quantified based on the drawings, as shown below: (distance between the right end of the object and that of the figure drawn by the subjects)/(distance between the left end of the object and the starting point)×100, where the denominator is always 50 mm.
All medical records were evaluated retrospectively. The baseline and clinical characteristics of the subjects were analyzed based on a medical history taken from the participants and their caregivers. Data included age, male-to-female ratio, years of education, history of present illness, current medications, presence of diseases that could impair cognitive function, family history of dementia, and presence of depression and other psychiatric diseases. This was followed by assessing the severity of dementia and cognitive impairment using the CDR. In addition, well-trained personnel presented the cognitive function tests (SNSB, MMSE, and GDS) and conducted a depression screening test.
VBM is a neuroimaging modality that examines changes in the brain by statistically analyzing brain MRI scans at the voxel unit level (a cube with six square faces whose height and width are equal). It was first used by Wright et al.17 in 1995 for patients with schizophrenia. It has been used to examine a variety of brain diseases, such as dementia.18
The MRI data were acquired by a 1.5-Tesla MRI (GE scanner, Signa Excite 11.0; General Electric Medical Systems, Milwaukee, WI, USA), and 170-180 slices of magnetization prepared rapid acquisition gradient echo T1-weighted images with a coronal 1-mm thickness were obtained (field of view=256×256 mm, number of excitations=1, repetition time=1780 ms, echo time=2.22 ms, flip angle=5°, with no interslice gap). The images were stored as Digital Imaging and Communication in Medicine files and reconstructed into three-dimensional Analyze format files using MRIcro. The VBM analysis was performed using SPM8 and MATLAB ver. 7.1 (Matlab Inc., Natick, MA, USA). The images were processed according to the following procedures: 1) spatial normalization was used, in which each image was fit to some template, 2) segmentation, in which each image was classified based on T1-weighted MR image intensity in the gray matter, white matter, and cerebrospinal fluid, and 3) modulation, in which voxel density is adjusted. These procedures were performed according to the differences in the shape of the voxels generated during spatial normalization to enhance the signal-to-noise ratio, which was followed by the VBM analysis using the gray matter images.19
We conducted a group comparison between the AD and the normal control groups using a two-sample t-test to determine significant regional differences in gray matter density between the two groups. The threshold for the result was set at a family-wise error (FWE)-corrected p-value<0.05 and an extended threshold of 100 voxels was applied. Covariate factors included were age, education level (expressed as years of education), and total intracranial volume (TIV).
We analyzed the closing-in phenomenon scores and the baseline characteristics of the two groups using the non-parametric Mann-Whitney test. This was followed by a multiple regression analysis using SPM8 interlocked with Matlab ver. 7.1. Thus, we used the closing-in phenomenon scores as the main variable and searched specific brain regions negatively associated with the closing-in scores in all subjects. Covariate factors included were age, education level (expressed as years of education), the MMSE, and TIV, each of which was adjusted for the analysis. The extent threshold was set at 100 at an uncorrected p-value<0.001. Thus, attempts were made to identify atrophied gray matter regions with more than a 100-voxel difference. The coordinates of the atrophied gray matter regions were entered into MNI/Talairach Coordinates in MRI-cro. Thus, the anatomical locations were identified accurately.
As shown in Table 1, we summarized such baseline and clinical characteristics as age, male-to-female ratio, and education level (years of education), as well as scores on the MMSE, CDR, and GDS in 38 patients with AD and 21 normal healthy controls. The MMSE and CDR scores were significantly lower in patients with AD than those in the controls. In addition, the patients with AD were significantly older but the GDS scores were significantly higher in normal healthy controls. No significant differences were observed for the male-to-female ratio (p=0.864) or education level between the groups.
We compared the closing-in phenomenon scores between the two groups (Fig. 2).
Overall, the mean closing-in phenomenon scores were significantly lower in patients with AD (66.2±23.0 vs. 91.2±9.5, p<0.0001). Based on the distribution of the percent grades, the patients with AD had closing-in phenomenon scores of 49.5, 71.0, and 82.0 points corresponding to the lower 25%, 50%, and 75%. These values were significantly lower than the 72.6, 88.0, and 93.0 points in the corresponding order in the normal healthy controls.
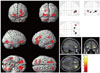
Atrophied gray matter was confirmed in the patients with AD (Fig. 3). To analyze the results, the extent threshold was set to 100 at an FWE corrected p-value<0.05. The results show atrophy in the right hippocampus, bilateral insula, and bilateral temporal lobes. In addition, a multiple regression analysis of the closing-in phenomenon scores in a single group of subjects comprised of the patients with AD and normal healthy controls was conducted (Table 2, Fig. 4). The results show that lower closing-in phenomenon scores were associated with progression of atrophy in bilateral orbito-frontal lobes.
We conducted this study to quantify the closing-in phenomenon that occurs in patients with AD and to objectively demonstrate atrophied gray matter regions in a VBM analysis. In particular, this is the first attempt to objectively quantify the closing-in phenomenon and identify the anatomical location of the gray matter using VBM analysis. This is in contrast to other studies that have examined the closing-in phenomenon mechanisms using neurocognitive function tests.
The closing-in phenomenon scores were significantly lower in patients with AD compared to those in the normal healthy controls, which agrees with previous reports that the closing-in phenomenon is a neurocognitive indicator of AD.1,6 The closing-in phenomenon is significantly severe in patients with AD but also occurs in normal healthy controls. We often seen a closing-in tendency in children and in 25% of normal elderly people, indicating that frontal lobe atrophy progresses with aging and could lead to the closing-in phenomenon which is presumed to be a primitive reflex.
The degree of the gray matter atrophy was significantly more severe in bilateral parieto-temporal lobes and the medial temporal lobe in patients with AD compared with that in the normal healthy controls, which agrees with previous reports. In addition, our results indicate that a VBM analysis is a reliable modality for assessing the closing-in phenomenon.20
Previous studies have mainly examined the closing-in phenomenon that occurs in patients with AD. Therefore, it is a matter of time when this phenomenon will be considered a symptom of AD. We performed a multiple regression analysis to identify the correlation between the closing-in phenomenon and atrophied gray matter regions in a single group of subjects comprising both groups. Thus, we mainly focused on the anatomical locations in the brain responsible for the closing-in phenomenon rather than the disease itself.
Of the hypotheses that have been proposed to explain the closing-in phenomenon, the compensation hypothesis explains that it occurs to compensate for visuo-spatial dysfunction. To support this hypothesis, Lee et al.9 reported that the closing-in phenomenon increasingly occurs when drawing a complex figure because there is increased demand on working memory.8,9,10 In addition, Luria16 compared executive and visuo-spatial frontal lobe functions in patients with AD and neurocognitive parameters. They reported that the closing-in phenomenon is correlated with the visuo-spatial functions rather than frontal lobe functions; thus, supporting the compensation hypothesis.21 In contrast, Ambron et al.22 reported that the closing-in phenomenon occurs increasingly when patients with AD attempt to draw a complex figure. These authors also found no correlation between the degree of complexity and closing-in in patients with fronto-temporal dementia (FTD). They explained that different factors may be involved in the occurrence of the closing-in phenomenon depending on the disease.23 The closing-in phenomenon reportedly occurs due to visuo-spatial dysfunction in patients with AD. However it has also been reported to occur due to the attraction behavior in patients with FTD. As additional evidence, Conson et al.24 reported that the degree of the closing-in phenomenon is independent of the visuospatial function in patients with corticobasal ganglia degeneration or focal frontal lobe lesions.22,24
In our study, the closing-in phenomenon was correlated relationship with atrophy of bilateral orbito-frontal areas, which supports the attraction hypothesis that the closing-in phenomenon occurs due to frontal lobe dysfunction as a part of a primitive reflex rather than the compensation hypothesis, which states that it occurs due to visuo-spatial dysfunction associated with the parietal lobe.
Visuo-spatial functions are needed to copy a complex figure. However, executive functions are also required because of the continuous concentration required on a complex figure.9
Damage to the orbito-frontal lobe triggers dis-inhibition, and the resulting impulse control disorder may lead to a sexual behavior disorder, overeating, alcoholism, heavy smoking, or drug abuse. In addition, obsessive behaviors or imitation and utilization disorders may also occur.25 The most notable of these is imitation behavior in which subjects imitate an observers' behavior. The closing-in phenomenon in which subjects attempt to overlap a drawing can be thought of as an imitation behavior. The anatomical location related to the attraction hypothesis would be the orbito-frontal lobe. The limitations of the current study are as follows:
1) This study was conducted solely in patients with AD. The closing-in phenomenon occurs frequently in patients with AD. Nevertheless, it is necessary to compare the pattern of occurrence of the closing-in phenomenon between patients with dementia who present with frontal lobe dysfunction, comprised of cases of FTD and subcortical vascular dementia, as well as those with AD.
2) This study was conducted solely in patients with AD who had mild-to-moderate symptom severity. Therefore, it is necessary to examine whether the closing-in phenomenon occurs in association with dementia progression in patients with more severe symptoms, including those with CDR scores of 2 or 3.
3) Discrepancies between the atrophied gray matter regions on the VBM analysis and the actual anatomical location may have occurred.25 Errors leading to distorted VBM results during normalization may have occurred within the limited frame in cases of severe cortical atrophy or severe hydrocephalus. Less severe atrophy than expected was found in parietal lobe shown in Fig. 3, and the frontal atrophy was more severe. These findings may be related to cognitive function of the study participants. Further study including comprehensive neuropsychological testing would make up for these limitations.
4) It is also probable that the images were classified incorrectly or their quality decreased during segmentation. However, we only enrolled patients with early-stage AD who had no severe cortical atrophy to minimize these errors. Attempts were also made to minimize the errors by directly confirming the images at each time point during the VBM analysis.
Despite these limitations, this study is significant, as were attempted to objectively quantify the closing-in phenomenon and identify the anatomical gray matter locations using VBM analysis of MRI images for the first time.
To summarize, we used VBM analysis to identify atrophied gray matter regions responsible for the occurrence of the closing-in phenomenon. Our results show that the degree of the closing-in phenomenon was associated with the progression of atrophy in bilateral orbito-frontal lobes. These results support the previously proposed attraction hypothesis.
Figures and Tables
Fig. 2
Percent distribution of the closing-in scores for patients with Alzheimer's disease (AD) and the normal controls

Fig. 3
Results of voxel-based morphometry t-test analysis comparing patterns of gray matter loss in the group of 38 patients with Alzheimer's disease (AD) with that of 21 healthy control subjects. Voxels showing significantly reduced gray matter volume in the patients with AD compared with the control group are indicated in red on a three-dimensional brain image (uncorrected p<0.05, left), in the gray scale on a glass-brain brain image (top right), and a template image with the color bar representing the t-statistic (corrected p<0.05, bottom right).
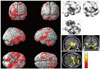
Fig. 4
Results of the voxel-based morphometry multiple regression analysis comparing patterns of gray matter loss associated with the closing-in phenomenon in 38 patients with Alzheimer's disease and 21 normal control subjects. Voxels show significantly reduced gray matter volume in the orbito-frontal areas in both groups (age and sex as covariates).
References
1. Gainotti G, Parlato V, Monteleone D, Carlomagno S. Neuropsychological markers of dementia on visual-spatial tasks: a comparison between Alzheimer's type and vascular forms of dementia. J Clin Exp Neuropsychol. 1992; 14:239–252.

2. Mayer-Gross W. Some Observations on Apraxia: (Section of Neurology). Proc R Soc Med. 1935; 28:1203–1212.
3. Muncie W. Concrete model and abstract copy: a psychobiological interpretation of the "closing-in" symptom of Mayer-Gross. J Nerv Ment Dis. 1938; 88:1–11.
4. de Ajuriaguerra, Muller M, Tissot R. [Apropos of some problems caused by apraxia in dementias]. Encephale. 1960; 49:375–401.
5. Gainotti G. A quantitative study of the "closing-in" symptom in normal children and in brain-damaged patients. Neuropsychologia. 1972; 10:429–436.

6. Gainotti G, Marra C, Villa G, Parlato V, Chiarotti F. Sensitivity and specificity of some neuropsychological markers of Alzheimer dementia. Alzheimer Dis Assoc Disord. 1998; 12:152–162.

7. McIntosh RD, Ambron E, Della Sala S. Evidence for an attraction account of closing-in behaviour. Cogn Neuropsychol. 2008; 25:376–394.

8. Kwak YT. "Closing-in" phenomenon in Alzheimer's disease and subcortical vascular dementia. BMC Neurol. 2004; 4:3.

9. Lee BH, Chin J, Kang SJ, Kim EJ, Park KC, Na DL. Mechanism of the closing-in phenomenon in a figure copying task in Alzheimer's disease patients. Neurocase. 2004; 10:393–397.

10. Chin J, Lee BH, Seo SW, Kim EJ, Suh MK, Kang SJ, et al. The closing-in phenomenon in Alzheimer's disease and vascular dementia. J Clin Neurol. 2005; 1:166–173.

11. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.

12. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of Expanded Clinical Dementia Rating (CDR) Scale. J Korean Neurol Assoc. 2001; 19:585–591.
13. Kang Y, Na DL. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co.;2003.
14. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356.

15. Christensen KJ, Moye J, Armson RR, Kern TM. Health screening and random recruitment for cognitive aging research. Psychol Aging. 1992; 7:204–208.

16. Luria AR. Human brain and psychological processes. New York: Harper & Row;1966.
17. Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995; 2:244–252.

18. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000; 11(6 Pt 1):805–821.

19. Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer's disease. Expert Rev Neurother. 2008; 8:1691–1702.

20. Yang DW, Hong YJ, Yoon BR, Shim YS, Kim YI. Clinical use of Voxel-based morphometry. In : Monthly meeting of the Korean Dementia Association; Seoul. 2009. 2.
21. Hänggi J, Streffer J, Jäncke L, Hock C. Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis. 2011; 26:719–734.

22. Ambron E, Allaria F, McIntosh RD, Della Sala S. Closing-in behaviour in fronto-temporal dementia. J Neurol. 2009; 256:1004–1006.

23. Serra L, Fadda L, Perri R, Caltagirone C, Carlesimo GA. The closing-in phenomenon in the drawing performance of Alzheimer's disease patients: a compensation account. Cortex. 2010; 46:1031–1036.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download