Abstract
Background
The described clinical characteristics of early-onset Alzheimer's disease (EOAD) are distinct from that of late-onset AD. We reported a patient with atypical EOAD.
Case Report
A 54-year-old, truck driver developed gradual visuospatial, language and calculation deficits for 3 months. The neuropsychological impairments were extensive. Brain MRI revealed asymmetric atrophy in left medial temporal lobe along with distinct widening of sylvian fissure. (18)F-florbetapir-positron emission tomography (PET) showed a high uptake in the cortex. Further, the profiles of cerebrospinal fluid (CSF) biomarker were compatible with AD.
Conclusions
We diagnosed the patient as EOAD based on the result of biomarker study. Increased Aβ burden was identified through amyloid PET imaging and decreased CSF Aβ level. The high rise of CSF Tau proteins was in agreement with the patient's extensive and rapid cognitive decline. This case report demonstrates the importance of AD biomarker study in confronting early-onset dementia with atypical clinical presentation.
Early-onset Alzheimer's disease (EOAD) is defined as the AD that developed before the age of 65-year-old.1 The arbitrary cut-off age of 65-year-old was based on the usual retirement age. However, the clinical characteristics of EOAD described are distinct from that of late-onset AD (LOAD). The early intense neuropsychiatric symptom and non-amnesic presentation are more frequent in EOAD.123
Recently, we experienced a case of atypical EOAD dementia in a patient who presented visuospatial deficit and language problem. Through stepwise evaluation of dementia, from the standard neuropsychological test, brain magnetic resonance imaging (MRI), (18)F-florbetapir positron emission tomography (PET), and the measurements of 3 established cerebrospinal fluid (CSF) AD biomarkers, we diagnosed the patient as AD with atypical clinical presentation. In this report, we described the clinical, laboratory, and radiologic characteristics of the patient to raise awareness of the recent issue related to the diagnosis of atypical EOAD.
A 54-year-old, right handed man visited our memory clinic complaining of inability to perform his daily job adequately. He had 9 years of formal education and was a product delivery truck driver for 20 years. He was good in a health without any diseases or trauma history except chronic alcohol intake for 30 years. From 3 months prior, the patient experienced gradual forgetfulness in loading products and problems in navigating to required destinations. He was found to frequently make mistakes in calculation for account book records. Therefore, he was recommended to resign from the company. Since then, he remained home bound or at his wife's store, because he easily got lost on unfamiliar roads. His family noticed that he became silent and the content of his speech was poor with use of limited words. Further, he was found awkward in using the cellular phone, avoided spontaneous use, and was often depressed.
On evaluation, he looked healthy, but was less concerned about his cognitive deficits when asked about his difficulties, in response to his family's report on his recent problems. He was the fifth child in his family. His mother had sudden death at 60-year-old due to unidentified cause, and his father suffered from unknown type of dementia since the age of 70 years. None of his siblings had any kinds of neurological disorders. On neurologic examination, his muscle tone, speed of fine movement, and gait were normal. Other abnormal neurologic signs were not evident. For further evaluation, we performed the comprehensive neuropsychological, and language test. He got 16 points in Korean Mini-Mental State Examination, lost scores in temporal orientation (-2), spatial orientation (-1), registration (-2), serial -7's test (-3), remote memory (-1), and stage command (-3), repetition (-1), and writing a complete sentence (-1). Seoul Neuropsychological Battery for the comprehensive cognitive evaluation.4 His cognitive performance was the far below the normal range in all cognitive domains that were validated by digit span, Korean version of Boston Naming Test (K-BNT), calculation, ideomotor praxis, Rey complex figure copy, Seoul Verbal Learning Test, recall of Rey complex figure, controlled oral association word recall test, and Stroop test (Table 1). On Korean version of Western Aphasia Battery for validation of language deficits, his speech was fluent, but circumstance not focused, repetition of syllable, paraphagic error, and mild stuttering were noted. His comprehension was slightly impaired, especially on sequential commands. However, the responses on yes/no question and auditory word recognition were good. The repetition was most seriously impaired with ability to repeat only up to 2 short phrases. The naming was decreased, as identified in K-BNT. The collective results were suggestive of conduction aphasia.5
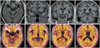
The blood chemistry, thyroid function test, and other laboratory test did not show other combined systemic disorder. Brain MRI revealed mild atrophy in the right side of medial temporal lobe, but marked atrophy in the left side (Fig. 1). The widening of sylvian fissure was evident in left side due to the diminished volume of adjacent temporal and inferior frontal and parietal lobe. And the sulcal widening of bilateral parietal lobe was also suspected. An (18)F-florbetapir-PET (Fig. 1) revealed a high uptake in the whole hemisphere. The stronger (18)F-florbetapiruptake was noted in bilateral parietal lobe.
Subsequently, the CSF AD biomarkers were checked after lumbar puncture under the informed consent from the patient and his family. The enzyme-linked immunosorbent assay using INNOTEST kit (Fujirebio, Ghent, Belgium) was used for analysis. The concentration of Aβ42, total Tau, and phospho-Tau181 (pTau181) in CSF was 323.3 pg/mL, 696.1 pg/mL, and 93.8 pg/mL, respectively. These fulfill the diagnostic criteria of AD.6 His apolipoprotein genotype was identified as ε3/ε3, and genetic study for causative mutation are planned. After diagnosis of AD, he has been on cholinesterase inhibitor and speech therapy for the past 2 months.
The dominant symptom of EOAD in our patient was distinct, demonstrating visuospatial, language and calculation deficits. His neuropsychological performance was extensively diminished involving all tested cognitive domains, and much worse than expected considering that his cognitive deficits became evident only 3 months prior. Although controversial, it is generally accepted that EOAD is more rapidly progressive and demonstrates atypical presentation compared with LOAD, as in our patient.1237 Thus, the AD diagnosis is often challenging in EOAD. The value of biomarker in AD diagnosis is proven and therefore incorporated into the revised diagnostic criteria of AD.89 Especially, the concentrations of Aβ42, tTau, and pTau181 in CSF are the currently superior AD biomarkers since they reflect the AD-related biochemical change of brain.101112 The international cooperative group has reached a consensus on the clinical usefulness of CSF biomarkers.13 Especially, 3 situations are suggested as an indication of the CSF biomarker analysis; early-onset dementia, mild cognitive impairments in case that the patient wants to know the result, and atypical clinical presentation.13 This indications were applicable to our patient who demonstrated early-onset dementia with atypical presentation. Among the dominant dementia symptoms, the conduction aphasia was the most unexpected feature of AD manifestation in our patient.14 The marked widening of left sylvian fissure due to the atrophy of the bordering structures is a relevant MRI lesion of the conduction aphasia.15 We diagnosed the case as AD with the help of AD biomarker study, amyloid imaging and CSF biomarker. The amyloid PET imaging and decreased CSF Aβ level indicated increased Aβ burden. In addition, the profiles of tau proteins were of diagnostic value. The markedly increased tTau and pTau181 level are indicative of ongoing severe axonal damage and increased tangle pathology in the patient's brain.10 These results were in agreement with his neuropsychiatric characteristics. The cognitive impairments were extensive and rapidly progressing. As reported in network study in EOAD,1617 wide destruction of functional network of the brain was suspected in our patient.
In summary, we recently diagnosed EOAD in a patient with the help of AD biomarker study, both Aβ imaging and CSF biomarkers. The atypical clinical characteristics of the patient involved various non-amnesic deficits from the earlier time of dementia. This case report emphasizes the importance of biomarker study in AD diagnosis, especially in early onset dementia with atypical clinical presentation.
Figures and Tables
Fig. 1
Neuroimaging studies. The axial T2-weighted fluid-attenuated inversion recovery image demonstrates left sided asymmetry in medial temporal atrophy (A). On the coronal 3D T1-weighted images, the asymmetric widening of left sylvian fissure (B and C), and bilateral parietal atrophy (D) are noted. The (18)F-florbetapir-PET reveals a high uptake in the whole hemisphere (E-H). The stronger uptake in the bilateral parietal lobes is suspected (G and H). PET: positron emission tomography.
References
1. Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD. The diagnosis of young-onset dementia. Lancet Neurol. 2010; 9:793–806.

2. Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early-versus late-onset Alzheimer's disease: more than age alone. J Alzheimers Dis. 2010; 19:1401–1408.

3. Panegyres PK, Chen HY. Differences between early and late onset Alzheimer's disease. Am J Neurodegener Dis. 2013; 2:300–306.

4. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076.

6. Park SA, Kim JH, Kim HJ, Kim TE, Kim YJ, Lee DH, et al. Preliminary study for a multicenter study of Alzheimer's disease cerebrospinal fluid biomarkers. Dement Neurocognitive Disord. 2013; 12:1–8.

7. Panegyres PK, Chen HY. Coalition against Major Diseases (CAMD). Early-onset Alzheimer's disease: a global cross-sectional analysis. Eur J Neurol. 2014; 21:1149–1154. e64–e65.

8. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014; 13:614–629.

9. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269.

10. Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med. 2012; 6:455–476.

11. Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008; 4:38–48.

12. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006; 5:228–234.
13. Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2014; 10:808–817.
14. Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2012; 27:413–420.

15. Mendez MF, Benson DF. Atypical conduction aphasia. A disconnection syndrome. Arch Neurol. 1985; 42:886–891.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download