Abstract
Background and Purpose
One of the most common genetic causes of frontotemporal dementia (FTD) is mutation in the progranulin (PGRN) gene. The aim of this study is to assess the early effects of the PGRN mutations on brain volumes by longitudinal voxel based morphometric (VBM) evaluation in asymptomatic mutation carriers.
Methods
We recruited 17 asymptomatic members of families with FTD caused by PGRN mutations; 7 mutation carriers (51.0±11.6 yr) and 10 non-carriers (55.2±6.0 yr, p=0.404). The MRI follow-up intervals of carriers and non-carriers were 788.6±103.8 and 922.0±225.1 days (p=0.124) respectively. We performed cross-sectional and longitudinal VBM analysis on both groups.
Results
At baseline, the carriers had lower white matter (WM) volumes in left frontal regions (p<0.001, uncorrected), but had no gray matter (GM) volume reduction. The carrier's global GM (p=0.924) and WM volume (p=0.364) reduction rate were not different from the non-carrier's. However, statistical parametric mapping T-maps showed differentially increased GM volume reductions in the bilateral parietal areas of carriers (p<0.001, uncorrected).
Conclusions
The findings from this study to examine WM and GM cross-sectional and longitudinal changes in PGRN mutation carriers suggest that WM atrophic changes could precede both GM changes and symptom onset in FTD. Asymptomatic PGRN mutation carriers have measurably higher rates of regional GM atrophy than non-carriers even in the pre-dementia stages.
Frontotemporal dementia (FTD) is the second most common cause of dementia in those under 65, and is clinically characterized by progressive behavior change, executive dysfunction and language difficulties. Approximately 40% of subjects have a family history and an autosomal dominant pattern of inheritance.12 The three most common mutations are the microtubule-associated protein tau gene,3 the progranulin (PGRN) gene,456 and the C9ORF72. A number of studies have characterized the imaging features of these mutation-carriers. Whitwell et al.7 assessed gray matter (GM) atrophy in FTD patients with PGRN mutations and compared them to FTD without PGRN mutations using voxel based morphometry (VBM) technique. They found that the PGRN carriers showed significantly more frontal and parietal GM loss than the PGRN non-carriers.
Other studies also implicated the parietal lobe as being more severely affected in PGRN mutation carriers.89 Clinical studies have also found deficits that result from the parietal lobe, such as limb apraxia, dyscalculia, visuospatial dysfunction and constructional disorders, are commonly seen FTD associated with PGRN mutations.1011
Since the patterns of atrophy in symptomatic PGRN carriers have now been relatively well characterized, an important next question is whether these patterns can be identified in subjects before they develop any symptoms of the disease. The evidence regarding GM loss in the asymptomatic PGRN mutation carriers is mixed. Borroni et al.12 investigated structural brain changes using VBM and diffusion tensor imaging (DTI) but found no differences in GM volume changes between the asymptomatic PGRN carrier and non-carrier group, although they found white matter (WM) changes in the left uncinate and inferior occipito-frontal fasciculus using DTI, suggesting that WM changes precede symptom onset. In contrast, Cruchaga et al.13 has found GM changes before onset of symptoms in PGRN carriers using VBM. GM volume loss was identified in the left frontal, temporal and parietal lobes and precuneus in the asymptomatic carriers.
The majority of VBM studies in FTD associated with PGRN mutation have been based on cross-sectional data, where consistent results have not been acquired especially in early or pre-symptomatic FTD subjects. The aim of this study is to assess the early effect of the PGRN mutation through the longitudinal VBM evaluation of pre-symptomatic PGRN carriers compared to non-carrier family members as controls.
We recruited 17 subjects, 7 of them were positive for mutations in PGRN and 10 of them were negative. All cases were family members of FTD patients diagnosed clinically and have at least one affected relative confirmed pathologically with frontotemporal lobar degeneration associated with TDP-43 inclusions. All study participants provided informed consent, and the study was approved by our institution ethics review board. There is no significant age difference between PGRN carriers and non-carriers (51.0±11.6, 55.2±6.0 yr, p=0.404). They had been prospectively studied in our University of British Columbia Hospital Clinic for Alzheimer and Related Disorders. They underwent annual standardized assessments including history collection, physical and neurological exams, blood tests, neuropsychological battery test, and a brain MRI.
At baseline, we did not identify any behavioral features, Parkinsonism, or significant language impairment. Neuropsychological battery tests were also normal (ref Brad's paper if in press). They also could do follow-up studies including neurological exams, neuropsychological battery test and a brain MRI. Each PGRN carrier and non-carrier's follow-up interval was 788.6±103.8 and 922.0±225.1 days (p=0.124).
MR studies were performed with a 1.5T machine (Philips, Amsterdam, the Netherlands) with a standardized imaging protocol that including a sagittal T1-weighted 3-dimensional volumetric spoiled gradient echo sequence with 166 contiguous partitions (inversion time: 800 ms; repetition time: 2000 ms; echo time: 20 ms; slice resolution: 0.5×0.5 mm; slice thickness: 0.5 mm).
VBM was used to assess group differences between the 7 PGRN carriers and 10 non-carriers implemented using VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) and statistical parametric mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm). All preprocessing procedures were performed according to the VBM8 manual.
We obtained the normalized global GM and WM volumes of baseline and follow-up images. The normalization of tissue volume was expressed by dividing the individual GM or WM volume by the total intracranial volume (TIV) and multiplying by 1000, a procedure that controls for variation in brain sizes across subjects. The TIV of each subject was obtained from the sum of GM, WM and CSF volume using the VBM8 toolbox.
To detect the GM or WM volume difference of PGRN carriers versus non-carriers, t-tests was conducted on the processed images, and age was treated as a covariate. This was performed in baseline and follow-up images by the SPM module of "Specify 2nd Level" at a statistical threshold of p<0.001 uncorrected for multiple comparisons.
To evaluate the longitudinal change of GM or WM volume over time of PGRN carrier, we apply the baseline and follow-up images of each subject onto the VBM8 toolbox that has a batch for longitudinal study design including intra-subject realignment, bias correction, segmentation and normalization. Each subject longitudinal data was registered to the baseline image of each subject.
We specified 2nd level using "Flexible Factorial" design with 17 subjects of the 2 groups, and 2 times. The interactions were defined between groups and times.
Age and follow-up interval were treated as covariates between study groups. The absolute threshold masking was 0.1. To detect the changes of tissue volume over time in PGRN carriers when comparing the changes in non-carriers, t-tests were conducted on the processed images.
Results were considered statistically significant at p<0.001 uncorrected for multiple comparisons.
There were no significant normalized global GM (p=0.311) and WM volume (p=0.313) differences between PGRN carriers and non-carriers in the baseline and follow-up images (Table 1). GM volume reduction rate was higher in the carriers than non-carriers, but was not statistically significant.
Statistical parametric maps of baseline and follow-up images also did not show the GM volume atrophy in PGRN carriers compared to non-carriers. However, there was significant regional WM volume reductions in the left frontal lobes in PGRN carriers (Fig. 1, uncorrected p<0.001).
The differential atrophic changes of regional GM volume associated with time were found in the right superior frontal lobules, bilateral parietal lobules (Fig. 2, uncorrected p<0.001). However, statistical parametric map did not show a significant longitudinal volume change of the WM between the carriers and non-carriers even in uncorrected p<0.01.
In this study, we analyzed GM volume changes over time in PGRN carriers at-risk for developing FTD compared to non-carriers. This is important to our understanding of the early changes in FTD progression. The major findings were, first, that baseline GM volume difference was not statistically shown between the PGRN carrier and non-carrier groups; second, that global GM volume of the carriers has a greater rate of atrophy than that of non-carriers, but not statistically significant; and third, that the carrier showed steeper regional tissue volume decrease particularly in the left frontal and bilateral parietal lobes.
As in previous studies,12 we found that asymptomatic PGRN mutation carriers did not show any significant GM atrophy at baseline, but we found that there is evidence of WM change in the left middle frontal lobes. It is suggest that WM change precede the GM and onset of clinical symptoms in FTD.
To identify PGRN related early neurodegeneration changes, we performed longitudinal analyze with repeated MRIs in PGRN carriers and non-carriers. Even though PGRN carriers and non-carriers showed very similar pattern of atrophic change with time, presumably as part of global aging, when we compared atrophic changes between the two groups, there is differential GM atrophic changes located in mainly the bilateral parietal lobes. These findings are similar to the GM changes reported in a previous study of symptomatic PGRN carriers.7
Although we did not obtain statistical significances, we found a tendency of higher volume reduction rate in the PGRN carrier group than non-carrier group.
It is readily assumable that even in asymptomatic subjects PGRN mutation related to reduction of cortical GM. PGRN mutations reduce progranulin levels and result in loss of function. Progranulin is a growth factor role in biological processes such as inflammation, wound healing, and cancer, and for its neurotrophic properties.14
In conclusion, this study suggests that WM changes could precede both of GM changes and symptom onset. Longitudinally, PGRN mutation has differential atrophic change on parietal areas even in at-risk FTD.
Figures and Tables
Fig. 1
Baseline of statistical parametric map of GM (A) and WM (B) regional volume reductions of frontotemporal dementia unaffected progranulin mutation carriers versus unaffected non-carrier. There is no significant GM atrophic area seen (controlled by age, uncorrected p<0.001) but WM reductions in left frontal area. GM: gray matter, SPM: statistical parametric mapping, WM: white matter.

Fig. 2
Statistical parametric map of longitudinal GM (A). Steeper GM changes were in bilateral middle parietal and right frontal areas (controlled by age and follow-up interval, uncorrected p<0.001). The changes of estimated marginal means of normalized GM volume of frontotemporal dementia unaffected PGRN carriers and non-carriers (B). GM: gray matter, PGRN: progranulin.
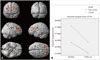
Table 1
Normalized global volumes of GM (nGM vol), WM (nWM vol), and cerebrospinal fluid (nCSF vol) of the PGRN carriers and non-carriers and their volume change rate

Each progranulin (PGRN) carrier and non-carrier's follow-up interval was 788.6±103.8 and 922.0±225.1 days (p=0.124).
The normalization of tissue volume was expressed by dividing the individual GM, WM, or CSF volume by the TIV and multiplying by 1000.
nCSF vol: normalized cerebrospinal fluid volume, nGM vol: normalized gray matter volume, nWM vol: normalized gray matter volume, TIV: total intracranial volume.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3331).
References
1. Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Arch Neurol. 1999; 56:817–822.

2. Rizzu P, Van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet. 1999; 64:414–421.

3. Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat. 2004; 24:277–295.

4. Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006; 442:916–919.

5. Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006; 442:920–924.

6. Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AM, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008; 131(Pt 3):721–731.

7. Whitwell JL, Jack CR Jr, Baker M, Rademakers R, Adamson J, Boeve BF, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol. 2007; 64:371–376.

8. Huey ED, Grafman J, Wassermann EM, Pietrini P, Tierney MC, Ghetti B, et al. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol. 2006; 60:374–380.

9. Rohrer JD, Warren JD, Omar R, Mead S, Beck J, Revesz T, et al. Parietal lobe deficits in frontotemporal lobar degeneration caused by a mutation in the progranulin gene. Arch Neurol. 2008; 65:506–513.

10. Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008; 131(Pt 3):732–746.

11. Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008; 131(Pt 3):706–720.

12. Borroni B, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C, et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res. 2008; 11:585–595.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download