Abstract
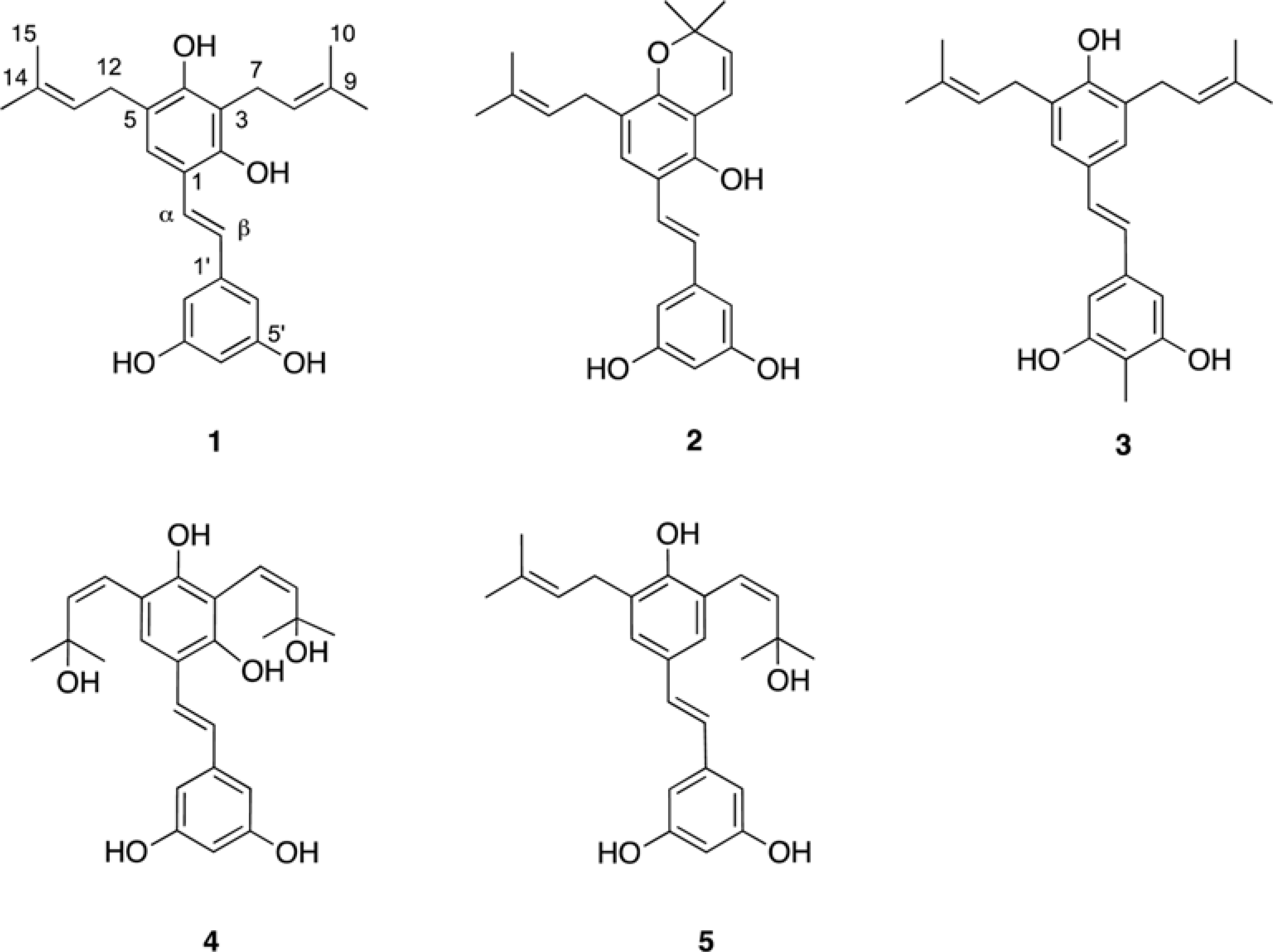
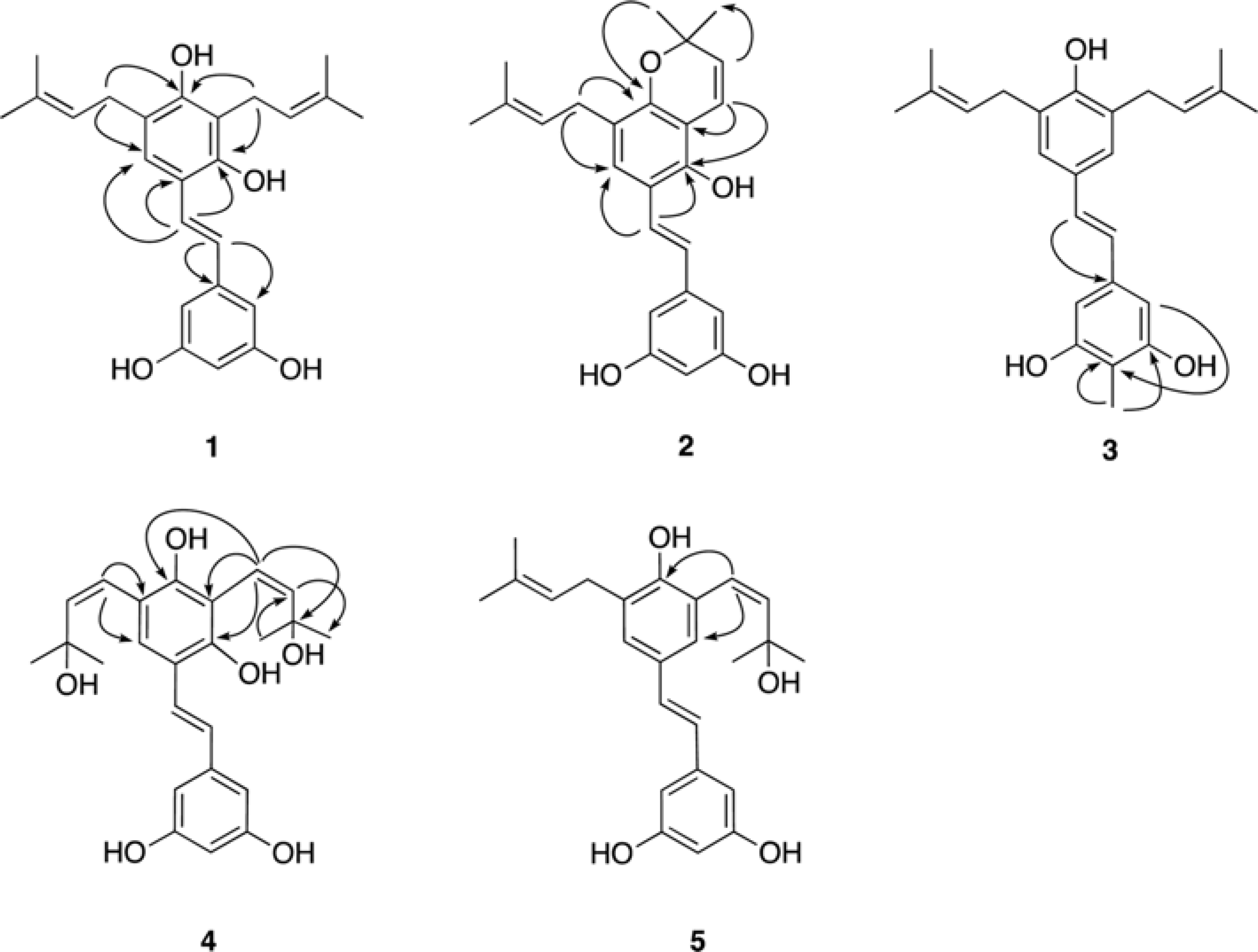
Five new prenylated stilbenes (1–5), along with the known compounds cudraflavone C, trans-4-isopentenyl-3,5,2′,4′-terahydroxystilbene, trans-4-(3-methyl-E-but-1-enyl)-3,5,2′,4′-tetrahydroxystilbene, pannokin G, cycloartobiloxanthone, artonin P, morusin, artocarpin, artonin E, kuwanon C, artobiloxanthone, and artoindonesianin C (6–17) were isolated from the stem bark of the tropical tree Artocarpus communis. The structures were established by NMR spectroscopic analysis, MS studies, and comparison with spectral data reported in the literature.
Go to : 
REFERENCES
(1). Jagtap U. B., Bapat V. A. J.Ethnopharmacol. 2010; 129:142–166.
(2). Sikarwar M. S., Hui B. J., Subramaniam K., Valeisamy B. D., Yean L. K., Balaji K. J.Appl. Pharm. Sci. 2014; 4:91–97.
(3). McKee T. C., Rabe D., Bokesch H. R., Grkovic T., Whitson E. L., Diyabalanage T., Van Wyk A. W. W., Marcum S. R., Gardella R. S., Gustafson K. R., Linehan W. M., McMahon J. B., Bottaro D. P. J.Nat. Prod. 2012; 75:1632–1636.
(4). Löfstedt T., Fredlund E., Holmquist-Mengelbier L., Pietras A., Ovenberger M., Poelinger L., Pahlman S.Cell Cycle. 2007; 8:919–926.
(5). Bokesch H. R., Gardella R. S., Rabe D. C., Bottaro D. P., Linehan W. M., McMahon J. B., McKee T. C.Chem. Pharm. Bull. 2011; 59:1178–1179.
(6). McCloud T. G.Molecules. 2010; 15:4526–4563.
(7). Toume K., Habu T., Arai M. A., Koyano T., Kowithayakorn T., Ishibashi M. J.Nat. Prod. 2015; 78:103–110.
(8). Pacher T., Seger C., Engelmeier D., Vajrodaya S., Hofer O., Greger H. J.Nat. Prod. 2002; 65:820–827.
(9). Kostecki K., Engelmeier D., Pacher T., Hofer O., Vajrodaya S., Greger H.Phytochemistry. 2004; 65:99–106.
(10). Hano Y.Heterocycles. 1990; 31:1339–1344.
(11). Takasugi M, Muñoz L., Masamune T., Shirata A., Takahashi K.Chem. Lett. 1978; 7:1241–1242.
(12). Boonlaksiri C., Oonanant W., Kongsaeree P., Kittakoop P., Tanticharoen M., Thebtaranonth Y.Phytochemistry. 2000; 54:415–417.
(13). Sultanbawa M. U. S., Surendrakumar S.Phytochemistry. 1989; 28:599–605.
(14). Hano Y., Inami R., Nomura T.Heterocycles. 1993; 35:1341–1350.
(15). Nomura T., Fukai T., Yamada S., Katayanagi M.Chem. Pharm. Bull. 1976; 24:2898–2900.
(16). Nomura T., Fukai T.Heterocycles. 1979; 12:1289–1295.
(17). Lin C. N., Lu C. M., Huang P. L.Phytochemistry. 1995; 39:1447–1451.
1451.(18) Sato M.., Fujiwara S.., Tsuchiya H.., Fujii T.., Iinuma M.., Tosa H.., Ohkawa Y. J.Ethnopharmacol. 1996. 54:171–176.
(19). Hano Y., Yamagami Y., Kobayashi M., Isohata R., Nomura T.Heterocycles. 1990; 31:877–882.
(20). Nomura T., Fukai T., Katayanagi M.Chem. Pharm. Bull. 1977; 25:529–532.
(21). Makmur L., Syamsurizal S., Tukiran T., Achmad S. A., Aimi N., Hakim E. H., Kitajima M., Takayama H. J.Nat. Prod. 2000; 63:243–244.
Go to : 
Table 1.
1 H NMR Assignments (600 MHz, CD3 OD) for compounds 1–5
Table 2.
13C NMR Assignments (150 MHz, CD3 OD) for compounds 1–5
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| position | δC, type | δC, type | δC, type | δC, type | δC, type |
| 1 | 119.6, C | 119.6, C | 130.8, C | 119.3, C | 131.2, C |
| 2 | 152.5, C | 149.9, C | 126.2, CH | 152.2, C | 123.2, CH |
| 3 | 118.8, C | 112.0, C | 130.1, C | 111.2, C | 122.6, C |
| 4 | 154.2, C | 152.2, C | 153.5, C | 149.6, C | 151.6, C |
| 5 | 122.5, C | 122.9, C | 130.1, C | 116.0, C | 130.4, C |
| 6 | 124.6, CH | 127.2, CH | 126.2, CH | 124.1, CH | 128.8, CH |
| 7 | 24.0, CH2 | 118.6, CH | 29.7, CH2 | 117.7, CH | 123.6, CH |
| 8 | 124.3∗, CH | 129.8, CH | 123.9, CH | 130.3, CH | 132.0, CH |
| 9 | 132.3, C | 76.3, C | 133.4, C | 77.5, C | 77.4, C |
| 10 | 18.0, CH3 | 27.9, CH3 | 18.0, CH3 | 28.2, CH3 | 28.2, CH3 |
| 11 | 26.0, CH3 | 27.9, CH3 | 26.0, CH3 | 28.2, CH3 | 28.2, CH3 |
| 12 | 29.4, CH2 | 29.1, CH2 | 29.7, CH2 | 123.2, CH | 29.4, CH2 |
| 13 | 124.2∗, CH | 124.8, CH | 123.9, CH | 129.4, CH | 124.1, CH |
| 14 | 133.2, C | 132.0, C | 133.4, C | 77.9, C | 132.7, C |
| 15 | 17.9, CH3 | 18.0, CH3 | 18.0, CH3 | 28.4, CH3 | 18.0, CH3 |
| 16 | 26.0, CH3 | 26.0, CH3 | 26.0, CH3 | 28.4, CH3 | 26.0, CH3 |
| α | 125.0, CH | 124.6, CH | 128.8, CH | 123.5, CH | 129.3, CH |
| β | 127.2, CH | 127.4, CH | 127.0, CH | 127.7, CH | 127.5, CH |
| 1′ | 141.9, C | 141.9, C | 137.4, C | 141.7, C | 141.2, C |
| 2′ | 105.8, CH | 105.8, CH | 105.5, CH | 105.7, CH | 105.8, CH |
| 3′ | 159.8, C | 159.6, C | 157.6, C | 159.7, C | 159.8, C |
| 4′ | 102.6, CH | 102.5, CH | 111.6, C | 102.6, CH | 102.8, CH |
| 5′ | 159.8, C | 159.6, C | 157.6, C | 159.7, C | 159.8, C |
| 6′ | 105.8, CH | 105.8, CH | 105.5, CH | 105.7, CH | 105.8, CH |
| 4′-CH3 | 8.6, CH3 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download