Abstract
The herbs of Lamium takesimense Nakai (Lamiaceae) is used to treat spasmodic and inflammatory disease. The four polar compounds, ecdysterone, isoacteoside, rutin and lamiuside C, were isolated and identified from the BuOH fraction of the L. takesimense MeOH extract. HPLC quantification was performed on a Capcell Pak C18 column (5 µm, 4.6 mm × 250 mm) with a gradient elution of H2O and 0.05% acetic acid in MeOH. The HPLC method was validated in terms of linearity, sensitivity, stability, precision, and accuracy. The quantitative level in plant material was determined as the following order: lamiuside C (4, 3.75 mg/g dry weight) > ecdysterone (1, 1.93 mg/g) > isoacteoside (2, 1.32 mg/g) > rutin (3, 0.97 mg/g).
Ecdysteroids are a group of hormonal steroids causing ecdysis in insects. Phytoecdysteroids refers to the ecdysteroid discovered in limited plants. The two terms, ecdysteroid and phytoecdysteroid, are named since they cause the ecdysis of insects. The most common phytoecdysteroids are ecdysone and ecdysterone which have the structure of polyhydroxylated steroids, and particularly α, β-unsaturated ketone in the B-ring. It was reported that those plants synthesize phytoecdysteroids for the purpose of a defense mechanism against insects. Weight loss, molting disruption and/or mortality are caused when insects take phytoecdysteroids.1 Phenylethanoid glycosides are a group of phenolic glycosides possessing phenylethyl and sugar moieties. It has been known that these substances have adaptogenic activities such as antioxidant, hepatoprotective, and anti-fatigue in addition to anti-Alzheimer's activity.23
Lamium takesimense belonging to the family Labiatae is said to have uterotonic, antispasmodic, and antiinflammatory, and to treat menstrual disorder in Oriental medicinal societies.45 The four compounds isolated from L. takesimense were identified as ecdysterone, isoacteoside, rutin, and lamiuside C. The HPLC experiments were performed to find how much they are contained in the BuOH fraction and the MeOH extract.
UV spectra were taken on a UV-visible recording spectrophotometer (Shimadzu, Japan). IR spectra were recorded on a JASCO 4200 FT-IR spectrometer with a KBr disk method. The Varian HPLC system used for a quantitative analysis consists of Prostar 210 pumps, a Prostar 325 UV-vis detector and a Shiseodo Capcell PAK C18 column (5 µm, 4.6 mm × 250 mm, Japan) equipped with its Meta Therm temperature controller. Solvents used for HPLC analysis were HPLC grade purchased from J. T. Baker (Phillisburg, NJ, USA). Stationary phases used for chromatographic isolation were silica gel 60 (70 – 230 mesh, Merck, Germany) and Sephadex LH-20 (GE Healthcare, Sweden).
The plant of L. takesimense (Lamiaceae) was collected at Mt. Chiak in Wonju, Gangwon-do, Korea, which was further dried and cut. This plant was identified by Prof. Byong-Min Song (Department of Forest Science, Sangji University). The voucher specimen was deposited in the Laboratory of Natural Products Chemistry, Department of Pharmaceutical Engineering, Sangji University.
The plant material of L. takesimense (358 g) was extracted under reflux for five hours three times. The extracted solution was filtered and evaporated under reduced pressure on a rotatory evaporator. The concentrated extract was further subjected to a freeze-drying to give a MeOH extract (46.0 g).
The MeOH extract was fractionated into the CHCl3 fraction as a less polar fractions and BuOH fractions as a more polar fraction. In brief, the MeOH extract was suspended in H2O and partitioned with CHCl3 three times. The CHCl3-soluble portion was concentrated and freeze-dried to give 14.0 g of CHCl3 fraction. A H2O-soluble portion was further fractionated with BuOH three times. The BuOH-soluble portion was concentrated and freeze-dried to give 9.0 g of the BuOH fraction.
To isolate constituents from the more polar fraction, the BuOH fraction (8.0 g) was subjected to column chromatography using a CHCl3-MeOH-H2O (65:35:10, lower phase) solvent and collected with each 60 ml volume (the fraction #1 – 50). The fractions were grouped into four fractions after checking TLC spots visualized by UV light or by 50%-H2SO4. As a result, the concentrated four fractions of LT-1 (#9–10), LT-2 (#24–26), LT-3 (#38–44), and LT-4 (#46–50) were produced. Compounds 1 and 2 were obtained by the purification of LT-1 and LT-2 with preparative TLCs, respectively. Compounds 3 and 4 were afforded by the purification of LT-3 and LT-4 with Sephadex LH-20 column chromatography, respectively.
Amorphous solid. UV λmax (MeOH) nm (log ε): 239 (3.95); IR νmax (KBr) cm−1: 3378 (broad, OH), 2939 (CH), 1701 (α,β-unsaturated ester), 1638, 1607 1514, 1447 (aromatic C=C), 1380, 1267, 1209 (phenolic C-O), 1057 (glycoside C-O), 879, 615; 1H-NMR (600 MHz, CD3OD) δ: 13C-NMR (150 MHz, CD3OD): (Agzmova et al., 2014)
Amorphous powder. UV λmax (MeOH) nm (log ε): 343 (3.78); IR νmax (KBr) cm−1: 3385 (broad, OH), 2932 (CH), 1701 (α,β-unsaturated ester), 1699 (α,β-unsaturated ester), 1607, 1508, 1457 (aromatic C=C), 1362, 1288 (phenolic C-O), 1175, 1075 (glycoside C-O), 815, 631; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD): (Schlauer et al., 2004)
Amorphous powder. UV λmax (MeOH) nm (log ε): 257 (4.08), 348 (4.00); IR νmax (KBr) cm−1: 3418 (broad, OH), 2934 (CH), 1657 (α,β-unsaturated ketone), 1600, 1506, 1456 (aromatic C=C), 1361, 1295, 1203 (phenolic C-O), 1061, 1015 (glycoside C-O), 808, 595; 1H-NMR (600 MHz, DMSO-d6) and 13C-NMR (150 MHz, DMSO-595; 1H-NMR (600 MHz, DMSO-d6) and 13C-NMR (150): (Park et al., 2004)
Amorphous powder. UV λmax (MeOH) nm (log ε): 333 (3.90); IR νmax (KBr) cm−1: 3389 (broad C-O), 2936 (CH), 1693 (α,β-unsaturated ester), 1630, 1608, 1519, 1449, 1364 (aromatic C=C), 1267 (phenolic C-O), 1116 (glycoside C-O), 818, 781, 631; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD): (Ito et al., 2006).
Four compounds isolated from L. takesimense were dissolved in MeOH to prepare standard stock solutions (1,000 µg/ml concentration) which were preserved in a refrigerator less than 4 ℃. The stock solution was serially diluted to prepare working standard solutions. Regression equations were determined by calculating the peak area (y) measured at six concentrations (x, µg/ml). To prepare sample solutions, the MeOH extract of L. takesimense, and its fractions (CHCl3- and BuOH fractions) were dissolved in MeOH. The sample solutions were filtered through adisposable syringe filter (0.50 µm, Sismic-25JP Advantec, Japan) prior to the injection to HPLC system.
The two solvents, H2O (solvent A) and 0.05% acetic acid in MeOH (solvent B), were used as the mobile phase. Gradient elution was performed as follows: 0 – 20 min (20 → 65% B), 20 – 21 min (65 → 100% B), 21 – 25 min (100% B), 25 – 27 min (100 → 20% B), and 27 – 30 min (20% B). The flow rate and column temperature was set constantly at 1.0 ml/min and 40 ℃, respectively. The detection wavelength was fixed at 254 nm and monitored during 30 min for each sample.
The HPLC method was validated in terms of linearity, sensitivity, precision, stability, and accuracy based on the guidance of ICH (International Conference on Harmonization). Linearity was evaluated by determining the R2 value of the equation obtained from the calculation of peak areas measured at the serially diluted six concentration. Sensitivity was assessed by determining the limit of detection (LOD) and limit of quantification (LOQ). LOD and LOQ were determined by the signal-to-noise (S/N) method, where 3 and 10 for S/N ratios were used for LOD and LOQ.
The precision and accuracy were assessed determining the intra-day and inter-day variabilities using the intermediate evaluation method. Intra-day variability was determined by measuring the sample solutions during a day (24 h). The inter-day variability was performed by injecting five times a day for consecutive four days. Relative standard equations (RSD) were determined by calculating the retention times and peak areas at the five repeated injection. RSD values were considered as the measure of precision and accuracy. The sample solution was spiked with each standard compound. Recovery rates were expressed by mean recovery rates (%) obtained from the calculation of spiked extract solution versus nonspiked extract sample.
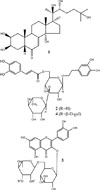
The four compounds isolated from the polar fraction (the BuOH fraction) were identified as ecdysterone,6 isoacteoside,7 rutin,8 and lamiuside C,5 comparing the spectroscopic data including 1H- and 13C-NMR with literatures. Ecydysterone (20-hydroxyecdysone) possessing the structure (2β,3β,5β,22R)-2,3,14.20,22,25-hexahydroxycholest-7-en-6-one belongs to phytoecdysteroids. The two phenylethanoid glycosides, isoacteoside and lamiuside C, have the structure 3,4-dihydroxyphenylethyl alcohol 8-O-α-Lrhamnopyranosyl-(1 → 3)-(6-O-trans-caffeoyl)-β-D-glucopyranoside (2) and 3,4-dihydroxyphenylethyl alcohol 8-O-β-D-galactopyranosyl-(1 → 2)-α-L-rhamnopyranosyl-(1 → 3)-(6-O-trans-caffeoyl)-β-D-glucopyranoside (4). Compound 3 was rutin with the structure of quercetin 3-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside (Fig. 1).
The HPLC method was optimized by considering the parameter of the mobile phase, gradient elution, column temperature and UV wavelength. As the mobile phase, H2O as the solvent A and MeOH as the solvent B were chosen because this solvent system exhibited a good resolution and acceptable peaks. Addition of 0.05% acetic acid in H2O is for the prevention of peak dispersion due to the ionization of phenolic substances. Various programs for gradient elution were examined for the better separation and the appearance of the peaks of four compounds on a chromatogram. The following gradient elution program was chosen: 0 – 20 min (20 → 65% B), 20 – 21 min (65 → 100% B), 21 – 25 min (100% B), 25 – 27 min (100 → 20% B), and 27 – 30 min (20% B), with the flow rate of 1.0mg/ml. The column temperature 40 ℃ was employed for the better separation and constant retention time.
Compound 1 was sensitive at a 254 nm wavelength, but not at other two wavelengths, 280 and 360 nm. Therefore, the wavelength 254 nm was used because the four compounds were sensitive at this wavelength. Validation experimentations on the present HPLC method were performed in terms of linearity, sensitivity, precision, stability, and accuracy. As shown in Table 1, R2 values of the regression equation obtained from six concentrations were more than 0.998, demonstrating that every equation is sufficiently linear.
Ecdysterone with a chromophore of α, β-unsaturated ketone in the B-ring absorbed a 254 nm wavelength, while common steroids were not sensitive at this wavelength. Limit of detection (LOD) and limit of quantification (LOQ) were determined to evaluate the sensitivity. LOD and LOQ were shown over 0.18 – 0.93 µg/ml and 0.59 – 3.10 µg/ml, respectively, demonstrating that this method is sufficiently sensitive for quantification. As shown in Table 2, the RSD values over the range 1.22 – 4.26% in the intra-day variability and 1.41 – 4.02% in the inter-day variability demonstrates the sufficient precision and stability. The recovery rates over the range of 101.07 – 104.91% stands for the accuracy of this HPLC method. Therefore, the present HPLC method was used for simultaneous quantitative determination of ecdysterone and other three phenolic substances in L. takesimense.
The four compounds were shown at the retention times of 12.03 min (lamiuside C), 14.82 min (isoacteoside), 13.30 min (rutin), and 15.58 min (ecdysterone) on the HPLC chromatogram, as shown in Fig. 2. The peaks of rutin and ecdysterone were pronounced on the chromatogram of the BuOH fraction. The content of four compounds analyzed by HPLC was shown in Table 3. By analyzing the MeOH extract, the quantitative level of four compounds in plant material was determined in the following order: lamiuside C (3.75 mg/g dry weight) > ecdysterone (1.93 mg/g) > isoacteoside (1.32 mg/g) > rutin (0.97 mg/g). These results suggest that lamiuside C was quantitatively highest of the four compounds. The CHCl3-and BuOH fractions obtained by fractionating the MeOH extract were also quantitatively analyzed. The quantities of four compounds were relatively higher in the BuOH fraction but lower in the CHCl3 fraction. In particular, the content of ecdysterone in the BuOH fraction was 267.15 mg/g BuOH fraction more than of lamiuside C (104.54 mg/g). A part of lamiuside C with three sugar moieties may have dissolved in the aqueous layer. These results demonstrate that ecdysterone was abundant particularly in the BuOH fraction.
It has been documented that the adaptogenic effect of many adaptogenic plant species covers anabolic, antioxidant, hepatoprotective, and hypoglycemic effects due to their content of phytoecdysteroid. Bathoriet al.9 reported the biological activity of phytoecdysteroids as anabolic steroids. The present HPLC analysis revealed that lamiuside C was highly contained in L. takesimense. Lamiuside C belonging to the phenylethanoid glycosidehas one more D-galactose than isoacteoside. Cistanche desrticola called desert ginseng has been known that its constituents of phenylethanoid glycosides have andaptogenic effects including neuroprotective, hepatoprotective, antioxidative and anti-fatigue activities.2 In addition, the neuroprotective activity of phenylethaoid glycosides due to the inhibition of amyloid β aggregation was also reported, indicating that they are effective against Alzheimer's disease.3 Therefore, the pharmacognostic use of L. takesimense could be sought on the basis of quantitative abundance of phytoecdysone and lamiuside C.
Figures and Tables
References
1. Dinan L. Phytochemistry. 2014; 57:325–339.
2. Wang T, Zhang X, Xie W. Am J Chin Med. 2012; 40:1123–1141.
3. Kurisu M, Miyamae Y, Murakami K, Han J, Isoda H, Irie K, Shigemori H. Biosci Biotechnol Biochem. 2013; 77:1329–1332.
4. Lee CB. Colored Flora of Korea. Seoul: Hyangmunsa;2014. p. 129.
5. Ito N, Nihei T, Kakuda R, Yaoita Y, Kikuchi M. Chem Pharm Bull. 2006; 54:1705–1708.
6. Agzmova MA, Isaev IMO, Mamathanov AU, Isaev MIO, Ibragimov TF. Adv Biol Chem. 2014; 4:1–4.
7. Schlauer J, Budzianowski J, Kukulczanka K, Ratajczak L. Acta Soc Bot Pol. 2004; 73:9–15.
8. Park HW, Baek NI, Kim SH, Kwon BM, Chung IS, Park MH, Kim SH, Kim DK. Korean J Pharmacogn. 2004; 35:320–323.
9. Báthori M, Tóth N, Hunyadi A, Márki A, Zádor E. Curr Med Chem. 2008; 15:75–91.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download