Abstract
By activity-guided fractionation, gliotoxin was isolated as an antibacterial metabolite of the fungus Penicillium decumbens which was derived from the jellyfish Nemopilema nomurai. Gliotoxin was further evaluated for antibacterial activity against several piscine and human MDR (multidrug resistance) pathogens. Gliotoxin showed significant antibacterial activity against Gram-positive piscine pathogens such as Streptococcus iniae FP5228, Streptococcus iniae FP3187, Streptococcus parauberis FP3287, Streptococcus parauberis SPOF3K, S. parauberis KSP28, and Lactococcus garvieae FP5245. Gliotoxin showed strong activity especially against S. parauberis SPOF3K and S. iniae FP5228, which are resistant to oxytetracycline. It is noteworthy that gliotoxin effectively suppressed streptococci which are the major pathogens for piscine infection and mortality in aquaculture industry. Gliotoxin also showed strong antibacterial activity against multidrug-resistant human pathogens (MDR) including Enterococcus faecium 5270 and MRSA (methicillin-resistant Staphylococcus aureus) 3089.
The ocean occupies 75% percent of the earth, and it provides a very special environment for marine microorganisms, which produce diverse secondary metabolites, and these secondary metabolites displayed various biological activities.1 Especially, endozoic microorganisms often produce interesting secondary metabolites since they occupy unique ecological niche unlike free-living marine microorganisms.2 Among endozoic microorganisms, such as bacteria, fungi, and microalgae, endozoic fungi are particularly recognized as potential resources for lead compounds for antimicrobial, antifungal, antioxidant, or anti-inflammatory agents.3
Enclosure aquaculture is one of the major sectors in the fishery industry of Korea. According to government statistics, aquaculture production was about 2.27 million tons, and its economic value was 3.84 billion USD during the first six months of 2017.4 However, mortality rate of high-density aquaculture is increasing due to microbial infections. Streptococci are the major bacterial pathogens for piscine infection and mortality in enclosure aquaculture. Regarding fish infectious diseases, 12.8% of fish death is caused by streptococcosis infection that is next only to the scuticociliatosis infection (35.9%). Because antibiotics such as oxytetracycline, amoxicillin, florfenicol, and oxolinic acid have been repeatedly used to treat these bacterial infections over long periods of time, antibiotic-resistant bacteria continuously appear.5 Accordingly, new antibiotics are required to counter this trend.
In our study for discovery of antibacterial compounds against piscine pathogens, gliotoxin (1) was isolated as an antibacterial metabolite from the jellyfish-derived fungus Penicillium decumbens. Subsequently, gliotoxin was evaluated for antibacterial activity against major piscine pathogens including streptococci and Gram negative bacteria. Gliotoxin exhibited significant antibacterial effect against streptococci including drug-resistant strains. Gliotoxin also effectively suppressed drug-resistant human pathogens MRSA 3089 and MDR Enterococcus faecium 5207.
Optical rotation was measured by using a JASCO DIP-1020 digital polarimeter. CD spectrum was recorded on a JASCO J-715 instrument in MeOH. 1D and 2D NMR spectra were recorded on Varian UNITY 400 and Varian INOVA 500 spectrometers. Chemical shifts were reported with reference to the respective residual solvent or deuterated solvent peaks (δH 3.30 and δc 49.0 for CD3OD). HR-MS data were obtained using an Agilent 1200 UHPLC accurate-mass Q-TOF/MS spectrometer. HPLC was performed using GILSON 307 pump with a Shodex packed C18M-10E column (250 × 10 mm, i. d. 5 µm) and a Shodex RI-101 detector.
The fungus Penicillium decumbens (J08NF-10) was isolated from the jellyfish Nemopilema nomurai which was collected from the southern coastal sea of Korea in August 2007. The sample (N. nomurai) was kept at the Marine Natural Product Lab, college of Pharmacy, Pusan National University, Korea. After the jellyfish was immediately cleaned by the seawater to remove non-attached microbes as soon as possible and quick to frozen at −20℃ in the refrigerating chamber, the fungi were isolated from the fresh jellyfish. Every small piece of tissue from the jellyfish were homogeneous to stir with the sterile seawater and cultured on the malt extract agar medium (MEA), which was prepared with containing glucose (20 g/L), agar (20 g/L), 75% seawater, malt extract (20 g/L), peptone (1 g/L), and antibiotics (final concentration: 50 µg/mL streptomycin and 50 µg/mL penicillin). Finally twelve pure fungal strains (J08NF-1 ~ J08NF-12) were isolated. The fungal strain J08NF-10 was selected on the basis of significant antibacterial activity against the human Gram-positive bacteria pathogen strain Staphylococcus aureus SG 511 (zone of inhibition 11 mm at 400 µg/disc), piscine Gram-positive pathogen strain Streptococcus iniae FP 5228. (zone of inhibition 11 mm at 400 µg/disc). The strain was identified as Penicillium decumbens by Dr. Kyung Sook Bae (Korea Research Institute of Bioscience & Biotechnology) using biochemical analyses.
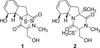
The fungal strain (P. decumbens) was cultured in malt extract agar plate containing 20 g of glucose, 20 g of malt extract, 1 g of peptone, and 20 g agar in 1 L of 75% seawater, and the strain (P. decumbens) was pre-cultured with 150 mL malt extract broth in 250 mL Erlenmeyer flask at 28℃ for 10 days, and then was transferred into 1 L large Erlenmeyer flask. Each containing 1 L of malt extract of this fungus (24 flasks, each 250 mL, Mass cultivation 24 L), P. decumbens was cultured by rotator shaker at 28℃ for 30 days. Finally the mass cultivation was extracted by the EtOAc, and then the liquid solvent was evaporated to dry by using the rotary evaporator. The crude extract was separated by using n-hexane and 90% MeOH. The 90% MeOH layer was subjected to a stepped-gradient MPLC (ODS-A, 120 Å, S-30/50 mesh) with 50% to 100% MeOH. Thirty fractions were obtained. These 30 fractions were also tested for antibacterial activity against the human Gram-positive pathogen Staphylococcus aureus SG 511 and piscine Gram-negative pathogen Vibrio ichthyoenteri FP 8487. Compound 1 (1.8 mg) and compound 2 (3 mg) (Fig. 1) were isolated on ODS HPLC (YMC ODS-H80, 250 × 10 mm, 4 µm, 80 Å) from the bioactive fraction 5 by using 45% MeOH-H2O.
white powder; [α]D26 : -246 (c 0.1, CHCl3); CD (c 0.1, MeOH) Δε (nm) -12.7 (224), 3.6 (277), 1H NMR (CD3OD, 500 MHz), δH 3.2 (3H, s, H-2), 4.81 (1H, d, J = 10.0 Hz, H-5a), 4.66 (1H, t, J = 10.0 Hz, H-6), 5.96 (1H, t, J = 10.0 Hz, H-7), 5.68 (1H, t, J = 10.0 Hz, H-8), 6.02 (1H, d, J = 10.0 Hz, H-9), 3.02, 3.72 (2H, d, J = 15.0 Hz, H-10), 4.33, 4.44 (2H, d, J = 15.0 Hz, H-11); 13C NMR (CD3OD, 100 MHz), δc 166.7 (C-1), 27.4 (C-2), 77.9 (C-3), 164.9 (C-4), 70.6 (C-5a), 74.8 (C-6), 130.4 (C-7), 124.3 (C-8), 120.8 (C-9), 131.5 (C-9a), 36.9 (C-10), 74.5 (C-10a), 60.6 (C-11); Q-TOF/MS m/z 325.0322 [M−H]−, calcd for C13H13N2O4S2 325.0322.
white powder; [α]D26 : -54.2 (c 0.1, MeOH); CD (c 0.1, MeOH) Δε (nm) -11.2 (224), 5.4 (277), 1H NMR (CD3OD, 500 MHz), δH 3.11 (3H, s, H-2), 4.92 (1H, d, J = 10.0 Hz, H-5a), 4.87 (1H, t, J = 10.0 Hz, H-6), 5.68 (1H, t, J = 10.0 Hz, H-7), 5.92 (1H, t, J = 10.0 Hz, H-8), 5.99 (1H, d, J = 10.0 Hz, H-9), 2.95, 3.11 (1H, d, J = 15.0 Hz, H-10), 3.87, 4.24 (1H, d, J = 10.0 Hz, H-11), 2.21 (3H, s, H-12), 2.27 (3H, s, H-13); 13C NMR (CD3OD, 100 MHz), δC 168.1 (C-1), 29.1 (C-2), 74.2 (C-3), 168.5 (C-4), 70.6 (C-5a), 75.8 (C-6), 130.8 (C-7), 124.8 (C-8), 120.9 (C-9), 133.7 (C-9a), 39.6 (C-10), 73.2 (C-10a), 64.6 (C-11), 13.6 (C-12), 15.2 (C-13); Q-TOF/MS m/z 357.0937 [M+H]+, calcd for C15H21N2O4S2 357.0937.
The piscine strains, S. parauberis FP 3287, Lactococcus garvieae FP 5245, S. iniae FP 5228 (oxytetracycline-resistant), Vibrio ichthyoenteri FP 8487 (oxytetracycline-resistant), Photobacterium damselae FP 4101, were provided by National Fisheries Research & Development Institute, Korea. S. parauberis KSP28, V. ichthyoenteri 0917-1, S. iniae FP 3187, and S. parauberis SPOF3K (oxytetracycline-resistant) were provided by Fish Disease Prevention Laboratory, Department of Aqualife Medicine, Pukyong National University, Korea. The methicillin resistant S. aureus 3089 (MRSA), MDR E. cloacae 0252, MDR E. coli 1137, MDR Enterococcus faecium 5207, and MDR Vibrio parahemolyticus 7001 were purchased from Korea National Research Resource Bank (KNRRB). All standard antibiotics were purchased from the Sigma Aldrich Co.
The minimum inhibitory concentration (MIC) is the lowest concentration of compound which prevents the visible growth of bacterium, and MIC value of the compound was determined using a 0.5 Mcfarland standard method. The compound was prepared in 0.5% DMSO-H2O in the range of 0.08 ~ 40 µg/mL. Concentration of antibiotics was also prepared to the range of 0.08 ~ 40 µg/mL by preparing in 0.5% DMSO-H2O. The turbidity of the bacterial suspensions was measured at 600 nm, and adjusted to 1 × 105 ~ 1 × 106 CFU/mL. In 96-well plates, 180 µL of bacterial culture and 20 µL of compounds solution were added to each well, and then, the plate was incubated at 37℃ for 24 h. The MIC values were determined by visual observation and optical density at 600 nm.
By activity-guided fractionation, diketopiperazine alkaloids (1 and 2) were isolated as antibiotic components from the jellyfish-derived fungus P. decumbens. Antibacterial activity against the human Gram-positive pathogen Staphylococcus aureus SG 511 and piscine Gram-negative pathogen Vibrio ichthyoenteri FP 8487 was monitored for fractionation. Compounds 1 and 2 were isolated from the active fraction (see experimental), and they were identified as gliotoxin and bisdethiobis (methylthio) gliotoxin, respectively (Fig. 1).
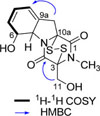
On the basis of Q-TOF/MS, 1H and 13C NMR, COSY, and HMBC data, the planar structure of compound 1 was defined to be the same as gliotoxin6 (Fig. 2).
Compound 1 showed negative specific rotation ([α]D23 : -246 in CHCl3), which is consistent with the reported data ([α]D24 : -255 in CHCl3) for gliotoxin.6 The absolute configuration of compound 1 was confirmed to be (3R,5aS,6S,10aR) by comparison of CD spectral data with those reported.6 The CD spectrum of compound 1 showed Cotton effects at 224 nm (Δε −12.7) and 277 nm (Δε +2.5) which are in the same pattern as the reported one (225 nm (Δε −7.5) and 278 nm (Δε +3.6)7, Fig. 3).
Considering possible biological role of gliotoxin in marine ecology, it was evaluated for antibacterial activity against piscine pathogens as well as human MDR pathogens (Table 1). Gliotoxin (1) showed significant antibacterial activity against Gram-positive piscine and human pathogens. Especially, compound 1 effectively suppressed oxytetracycline-resistant S. iniae FP5228 and S. parauberis SPOF3K. Bacterial infection by streptococci (sterptococcosis) is the major cause of mortality in fish culture, and oxytetracycline is frequently employed as an antibiotic in enclosure aquaculture. It was found that gliotoxin (1) show good antibacterial activity against not only to oxytetracycline-resistant piscine pathogens, but also to drug-resistant human pathogens, methicillin-resistant Staphylococcus aureus (MRSA) 3089 (MIC : 0.63 µg/mL) and MDR Enterococcus faecium 5270 (MIC : 0.32 µg/mL).
Compound 2 was identified as bisdethiobis (methylthio) gliotoxin by comparison of NMR, CD, and specific rotation data with those reported.8 Bisdethiobis (methylthio) gliotoxin (2) was previously isolated from a marine-derived fungus of the genus Pseudallescheria, and it's weak antibacterial activity against the methicillin-resistant and multidrug resistant Staphylococcus aureus was reported.8 In this study, compound 2 showed weak inhibitory activity against the piscine pathogen S. parauberis FP 3287 with the MIC value of 20 µg/mL.
It is noteworthy that gliotoxin (1) showed potent antibacterial activity against the oxytetracycline-resistant piscine pathogens S. iniae FP 5228 and S. parauberis SPOF3K. Gliotoxin also effectively suppressed drugresistant human pathogens MRSA 3089 and MDR Enterococcus faecium 5207. Previously, gliotoxin was reported to be antibiotic to human bacterial pathogens such as Escherichia coli, Aerobacter aerogenes, Pneumococcus Types I, III, and Staphylococcus aureus (R3708).6 Antibacterial activity of gliotoxin against piscine pathogens is first reported in this study, and significant antibacterial activity against human MDR pathogens Enterococcus faecium 5207 and MRSA 3089 was not previously reported. Gliotoxin was often reported for significant cytotoxicity against tumor cells,9 and its substantial acute toxicity to hamsters was revealed.10 Therefore, further study on gliotoxin derivatives should be aimed for optimization of antibacterial activity against drug-resistant pathogens while minimizing its toxicity.
Acknowledgments
This study was supported by a 2-year research grant of Pusan National University. The piscine pathogens were kindly donated by National Fisheries Research and Development Institute, Busan, Korea, and Fish Disease Prevention Laboratory, Department of Aqualife Medicine, Pukyong National University, Korea.
References
1. Zhang HW, Song YC, Tan RX. Nat Prod Rep. 2006; 23:753–771.
2. Bugni TS, Ireland CM. Nat Prod Rep. 2004; 21:143–163.
3. Zhang Y, Mu J, Feng Y, Kang Y, Zhang J, Gu PJ, Wang Y, Ma LE, Zhu YH. Mar Drugs. 2009; 7:97–112.
4. Ingavat N, Dobereiner J, Wiyakrutta S, Mahidol C, Ruchirawat S, Kittakoop P. J Nat Prod. 2009; 72:2049–2052.
5. Jee BY, Shin KW, Lee DW, Kim YJ, Lee MK. J Fish Pathol. 2014; 27:77–83.
6. Aoki T, Arai T, Egusa S. Microbiol Immunol. 1977; 21:77–83.
7. Johnson John R, Bruce William F, Dutcher James D. J Am Chem Soc. 1943; 65:2005–2009.
8. Nagarajan R, Woody RW. J Am Chem Soc. 1973; 95:7212–7222.
9. Li X, Kim SK, Nam KW, Kang JS, Choi HD, Son BW. J Antibiot (Tokyo). 2006; 59:248–250.
10. Vigushin DM, Mirsaidi N, Brooke G, Sun C, Pace P, Inman L, Moody CJ, Coombes RC. Med Oncol. 2004; 21:21–30.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download