Abstract
During the screening for cytotoxic compounds from plants grown in Korea, Betula platyphylla (BP) showed potent activity against the adenocarcinomic human alveolar basal epithelial A549 cell line. To identify the cytotoxic components from BP, the CH2Cl2 fraction with the most significant cytotoxic effect was applied to the column chromatographies. Seven compounds were isolated: lupeol (1), betulinic acid (2), (−)-rhododendrol (3), platyphyllenone (4), platyphyllone (5), (−)-centrolobol (6), and oleanolic acid (7). Among them, three diarylheptanoids (4 – 6) exhibited cytotoxicity toward A549 cells. Especially, 50 µM of 4 reduced A549 cell viability to 18.93 ± 0.82% compared to control (100.00 ± 21.48%). Lactate dehydrogenase (LDH) leakage and intracellular reactive oxygen species (ROS) production were also induced by 50 µM 4. This is the first report on the cytotoxic effect of BP-derived diarylheptanoids 4–6 against A549 cells. The compound 4 may be useful for the development of early hit compounds for non-small cell lung carcinoma, but the consideration about selectivity of 4 is required since 4 also showed the cytotoxicity in the human normal lung epithelial BEAS-2B cell line.
Lung cancer is the main cause of cancer-related mortality worldwide, and its incidence is still rapidly increasing.12 Based on histological characteristics, the lung cancer is divided into two subtypes: small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC)1; in particular, NSCLC occurs in approximately 85% of all lung cancer cases.3 In addition, surgical resection cannot be applied for NSCLC initially diagnosed at stages III or IV, and the 5-year survival rate is less than 5% for advanced NSCLC.34 Therefore, the development of anti-NSCLC agents is required.
Various drugs, including cisplatin, paclitaxel, docetaxel, gemcitabine, have been used for chemotherapy against NSCLC.5 However, they commonly induced the side effects include hair loss, mouth sores, vomiting, diarrhea, and fatigue, as the drugs attack both cancer cells and normal cells.6 Owing to the disadvantages of chemotherapy, the targeted therapy has been investigated. Targeted therapy is a highly specific therapeutic approach to target the tumor cells and increase the survival rate of patients.7 Specific agents for targeting angiogenesis (bevacizumab and ramucirumab), the epidermal growth factor receptor (EGFR; erlotinib, afatinib, and gefitinib), abnormal anaplastic lymphoma kinase (ALK) protein (crizotinib, ceritinib, alectinib, and brigatinib), and v-raf murine sarcoma viral oncogene homolog B (BRAF; dabrafenib) have been developed.7 Alternatively, the immunotherapy has been considered, which functions through the stimulation of the patient's own immune system to eliminate the tumor by using nivolumab.6 However, both targeted therapy and immunotherapy still show common or serious side effects.67
In contrast to the previously mentioned drugs, natural products offer various advantages, including comparative non-toxicity, abundant availability, cost-effectiveness, and multi-target functions.7 Therefore, a variety of natural compounds have been screened, which are effective in the treatment of lung cancer in vitro or in vivo, such as 8-epi-xanthatin (a sesquiterpene lactone) from Xanthium strumarium, hederacolchicoside A 1 (a triterpenoid) from Hedera colchica, methyl rocaglate (a lignan) from Aglaia formosana, and antofine (an alkaloid) from Cynanchum paniculatum.8 Especially, gossypol, a polyphenol from Gossypium hirsutum,9 which is a pan Bcl-2 inhibitor, has been used in clinical trials for SCLC1011 and NSCLC.12
During the screening for cytotoxic components from Korean plants, Betula platyphylla (BP, Asian white birch) showed strong activity on A549 cells. BP, belonging to the Betulaceae family, has been used as a traditional medicine in Asia for the treatment of pneumonia, nephritis, and chronic bronchitis.13 Various chemical constituents of BP have been identified, including platyphyllenone, platyphylloside,14 platyphyllin A,15 betulin,16 betulinic acid, oleanolic acid, and chilianthin A – C.17 It has been reported that the compounds derived from BP showed various biological activities, such as anti-oxidative,1718 anti-fibrotic,19 hepatoprotective,18 and anti-adipogenic20 effects. However, except for two recent reports,1321 the effects on lung cancer activity have been rarely reported.
Based on these backgrounds, we attempted to identify natural cytotoxic compounds from BP that could reduce the survival of adenocarcinomic human alveolar basal epithelial A549 cells, a NSCLC cell line.
The organic solvents, including methanol (MeOH), n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (n-BuOH), were obtained from Duksan Chemical (Anseong, Korea). Purification of the compounds was performed by column chromatography by using silica gel (70 – 230 mesh, Merck #7734, Darmstadt, Germany) and octadecyl silica (ODS, 150 µm, YMC #AA12SA5, Kyoto, Japan). Pre-coated thin-layer chromatography (TLC) silica gel 60 F254 (Merck #1.05554.0001) and TLC silica gel 60 RP-18 F254S (Merck #1.05559.0001) were used to monitor the eluted compounds. Nuclear magnetic resonance (NMR) was performed on a Bruker Avance Digital 500 NMR spectrometer (Karlsruhe, Germany). The NMR solvents (CDCl3 and CD3OD) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The absolute configuration was determined by a Jasco P-2000 polarimeter (Tokyo, Japan).
The dried stems of BP grown in Korea were purchased in 2015 at the local market in Daegu, Republic of Korea, and identified by Prof. Kyung-Sik Song. A voucher specimen (#KNUNPM BP-1) was deposited at the Laboratory of Natural Products Medicine, College of Pharmacy, Kyungpook National University.
BP (10 kg) was refluxed three times in MeOH for 6 h. The extracted solution was filtered and concentrated to obtain the MeOH extract (321.4 g). The MeOH extract was suspended in distilled water and successively partitioned with n-hexane, CH2Cl2, EtOAc, and n-BuOH. After evaporation, the fractions soluble in n-hexane (9.9 g), CH2Cl2 (49.6 g), EtOAc (42.8 g), and n-BuOH (35.6 g) were obtained. The CH2Cl2-soluble fraction was applied to a silica gel column (9 × 82 cm; CH2Cl2-MeOH, 500:1 → 1:1) to yield 25 fractions. Compounds 1 (51.4 mg) and 2 (76.0 mg) were obtained from fractions 2 and 5, respectively. Fraction 7 was rechromatographed on an ODS column (489.6 mg; 3 × 30 cm; water-MeOH, 7:3 → 1:9; flow rate 15 mL/min) to isolate compounds 3 (118.0 mg) and 4 (27.5 mg). Compounds 5 (20.0 mg), 6 (7.0 mg), and 7 (17.0 mg) were purified from fraction 11 (288.3 mg; 3 × 30 cm; water-MeOH, 4:1 → 1:9; flow rate 15 mL/min) by ODS medium pressure liquid chromatography (MPLC).
The human alveolar adenocarcinoma cell line A549 and a human normal epithelial cell line BEAS-2B were purchased from Korean Cell Line Bank (Seoul, Korea) and American Type Culture Collection (ATCC, Manassas, VA, USA), respectively. The cell culture medium used was Roswell Park Memorial Institute (RPMI) 1640 (Welgene, Gyongsan, Korea) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) (v/v), 100 U/mL penicillin, and 100 µg/mL streptomycin (Welgene). The cells were cultured at 37 ℃ in an atmosphere of 5% CO2. Subculturing was performed every 48 h.
To examine the cytotoxicity of the test samples on A549 and BEAS-2B cells, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Biosesang, Seongnam, Korea) assay was performed. The cells were seeded at a density of 2 × 104 cells/mL on a 48-well plate, incubated for 24 h, and the test sample was applied to the cells for 48 h. Subsequently, the media was removed, and 0.2 mg/mL MTT in phenol red-free and serum-free RPMI1640 medium was added. The insoluble formazan crystals produced by the live cells were dissolved in dimethyl sulfoxide after the removal of MTT solution. The optical density (OD) was measured at 545 nm by using a microplate reader. The cell viability was expressed as a percentage relative to the OD of the vehicle-treated control. For comparison, the anti-cancer reagent 5 µM doxorubicin (DOX; Sigma-Aldrich) was used as a positive control.
The cytotoxicity of the test sample on A549 cells was additionally evaluated through the use of a lactate dehydrogenase (LDH) release assay kit (CytoTox96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI, USA) in accordance with the manufacturer's instructions. The supernatant and the cell lysate were prepared after the cell treatment described in section of MTT cell viability assay. The OD value was measured at 490 nm. The LDH leakage was calculated as follows:
LDH leakage (fold) = A/(A + B)
A, LDH in supernatant; B, LDH in cell lysate
A549 cells were seeded at a density of 2 × 105 cells/mL in a 6-well plate. After 24 h, the cells were treated with test samples for 24 h. The cells were detached from the plate using 0.25% trypsin/ethylenediaminetetraacetic acid (EDTA; Welgene), washed once with phenol red-free and serum-free RPMI1640 medium, and collected. The collected cells were incubated with 5 µM of 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate and acetyl ester (CM-H2DCFDA; Invitrogen, Eugene, OR, USA) for 15 min in the dark. The cells were washed once and resuspended in phenol red-free and serum-free RPMI1640 medium before flow cytometric analysis of 104 cells was conducted on a FACS ARIA III (BD, San Diego, CA, USA).
The A549 cell viability was significantly reduced to 45.83 ± 3.65% after treatment with 200 µg/mL MeOH extract of BP compared to control (CNT, 100.00 ± 23.12%; Fig. 1). Next, the four fractions obtained from BP were used to treat A549 cells: 100 µg/mL of the n-hexane and CH2Cl2 fractions decreased cell viability to 56.10 ± 11.99% and 52.76 ± 3.14%, respectively, whereas the EtOAc and n-BuOH fractions were not effective in reducing of cell viability. As the CH2Cl2 fraction was the most effective (Fig. 2), it was selected for further experiments.
Seven compounds were isolated from the CH2Cl2 fraction and identified as lupeol (1),2122 betulinic acid (2),23 (−)-rhododendrol (3),2425 platyphyllenone (4),26 platyphyllone (platyphyllonol, 5; obtained as a racemic mixture),27 (−)-centrolobol (6),2728 and oleanolic acid (7)2930 through the comparison of their NMR data with those of previous studies (Fig. 3) in which compounds 1 – 7 were identified as the constituents of BP.14313233
As shown in Fig. 4, the treatment with 2 and 4 – 7 at 50 µM resulted in a less than 50% survival rate of A549 cells compared to the control (100.00 ± 23.12%). In contrast, 1 and 3 did not reduce A549 cell viability to less than 50%, even at 50 µM, as described in previous reports.34353637 Compounds 2 and 7 decreased the cell viability to below 50% at 5 µM (30.49 ± 1.95% and 43.84 ± 2.62%, respectively). Compounds 238 and 734 have been previously examined for cytotoxicity against A549 cells. In these studies, it was shown that the concentration that resulted in 50% cytotoxicity (CC50) was 43.4 µM after treatment with 2 for 72 h38 and 52.97 ± 1.22 µg/mL (approximately 116.16 µM) after treatment with 7 for 48 h.27 This difference might be a result of the cell concentrations used. At 50 µM, 4 reduced A549 cell viability to 18.93 ± 0.82%. In addition, 5 (46.54 ± 2.02%) and 6 (42.15 ± 1.13%) showed moderate cytotoxicity to A549 cells at 50 µM. The cytotoxic effects of 4 on the human NSCLC cell line NCI-H460 (CC50, 8.2 ± 0.1 µM) has been reported.39 In addition, 5 and 6 effectively induced cytotoxicity in the human NCI-H187 SCLC cell line (CC50 = 59.24 and 9.55 µM, respectively).40 However, to the best of our knowledge, this is the first report about the cytotoxic effects of 4 – 6 on A549 cells. Among the three compounds 4 – 6, 4 was the most effective and was applied to further experiments.
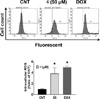
After treatment with 4, the cytosolic lactate dehydrogenase (LDH) leakage in A549 was induced in a concentration-dependent manner. In particular, the LDH leakage in the group treated with 50 µM 4 was 7.50 ± 0.50-fold greater compared with that of the control (CNT, 1.00 ± 0.04-fold; Fig. 5). Moreover, the production of intracellular reactive oxygen species (ROS) was significantly increased by 3.86 ± 1.05-fold by the treatment of 50 µM 4 compared with CNT (1.00 ± 0.12-fold; Fig. 6).
To determine the selectivities of compounds, the effects of compounds on the viability of human normal epithelial cell line BEAS-2B were examined. As shown in Fig. 7, compounds 1 and 3 did not show any cytotoxicities at the concentrations of 1–50 µM, although they revealed the reduction of A549 cell viability. In contrast, the viabilities of BEAS-2B cells were decreased to below 50% by 2 (20.53 ± 1.27%), 4 (13.55 ± 0.37%), and 5 (45.40 ± 4.07%) at 50 µM relative to the control (100.00 ± 3.78%). In particular, the reduction pattern of BEAS-2B cell viability induced by the most A549 cytotoxic compound 4, was similar to that of A549. Therefore, the selectivity of 4 for lung cancer cells has to be considered to develop the hit compounds. To the best of our knowledge, the effects of 1 – 6 on the BEAS-2B cell viability were firstly examined in this study, except for 7.41
The diarylheptanoid platyphyllenone (4) is a constituent of not only BP,16 but also Alnus japonica,4243 A. viridis,44 A. hirsute,45 and A. nepalensis.46 In a previous report, 4 showed anti-influenza activity against KBNP-0028 (H9N2) avian influenza virus, with a 50% effective concentration (EC50) of 29.9 ± 2.5 µM.43 Additionally, 4 was reported to inhibit the proliferation of the human pancreatic ductal carcinoma cell line PANC-1 (CC50= 6.06 µM) through the modulation of the shh-Gli-FoxM1 pathway.42 The anti-microbial activity47 of 4 has been also reported, however, other biological activities have been rarely investigated. Especially, there is only one report of the anti-lung cancer effect of 4 against NCI-H460 cells.39
The evaluation of the structure–activity relationships (SAR) by Novakoviæ et al. (2014)48 and Diniæ et al. (2016)39 revealed the structural aspects responsible for the cytotoxic effects of diarylheptanoids against NCI-H460 cells: i) a carbonyl group (C=O) at the C-3 position; ii) a double bond between C-4 and C-5, instead of a C-5 substitution with -OCH3, -Oxyl, -OGlc, and -OH; iii) the absence of the-OH groups at C-3 and C-3.3948 A549 is a NSCLC cell line, for which considerable information about SAR is already known.39,48 The most effective diarylheptanoid 4 also satisfied the above three conditions, which suggested the importance of the positions of the carbonyl and hydroxyl groups and the double bond. This study is the first to report about the cytotoxic effects of 4 – 6 on the human NSCLC cell line, A549. Further studies are required to determine the value of 4 as a potential drug lead for NSCLC.
Figures and Tables
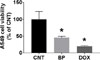 | Fig. 1The cytotoxic effects of Betula platyphylla (BP) extract (200 µg/mL) on A549 cells. Doxorubicin (DOX; 5 µM; A549 cell viability, 19.69 ± 2.84%) was used as a positive control. *, p < 0.05 compared with the control group (CNT). |
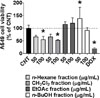 | Fig. 2The cytotoxic effects of the partitioned fractions from BP on A549 cells. DOX (5 µM; A549 cell viability, 20.57 ± 2.62%) was used as a positive control. *, p < 0.05 compared with CNT. |
 | Fig. 4The cytotoxic effects of isolated compounds 1 – 7 from BP on A549 cells. DOX (5 µM; A549 cell viability, 28.39 ± 6.21%) was used as a positive control. *, p < 0.05 compared with CNT. |
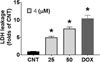 | Fig. 5The lactate dehydrogenase (LDH) leakage induced by platyphyllenone (4) in A549 cells. DOX (5 µM; LDH leakage, 10.46 ± 0.90-fold) was used as a positive control. *, p < 0.05 compared with CNT. |
Acknowledgements
This study was supported by National Institute of Biological Resources (NIBR) from Ministry of Environment (#NIBR201527101, Republic of Korea).
References
1. Zhu QG, Zhang SM, Ding XX, He B, Zhang HQ. Oncotarget. 2017; 8:57680–57692.
2. Dong L, Lei D, Zhang H. Oncotarget. 2017; 8:64600–64606.
3. Wang J, Chen J, Guo Y, Wang B, Chu H. Oncotarget. 2017; 8:53854–53872.
4. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2016; 66:7–30.
5. Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N, Kougioumtzi I, Karapantzos I, Huang H, Spyratos D. J Thorac Dis. 2013; 5:S4. S389–S396.
6. Kroschinsky F, Stölzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, Schellongowski P. Crit Care. 2017; 21:89.
7. Daga A, Ansari A, Patel S, Mirza S, Rawal R, Umrania V. Asian Pac J Cancer Prev. 2015; 16:4147–4156.
8. Ancuceanu RV, Istudor V. Altern Med Rev. 2004; 9:402–419.
9. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H. Biotechnol Adv. 2015; 33:1582–1614.
10. Schelman WR, Mohammed TA, Traynor AM, Kolesar JM, Marnocha RM, Eickhoff J, Keppen M, Alberti DB, Wilding G, Takebe N, Liu G. Invest New Drugs. 2014; 32:295–302.
11. Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan R. J Thorac Oncol. 2011; 6:1757–1760.
12. Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, Wood BA, Leopold L. J Thorac Oncol. 2011; 6:781–785.
13. Eom HJ, Kang HR, Choi SU, Kim KH. Chem Biodivers. 2017; 14.
14. Jiang H, Shen Y, Yasuda E, Chiba M, Terazawa M. Eurasian J For Res. 2001; 3:49–54.
15. Wang SJ, Pei YH, Hua HM. J Asian Nat Prod Res. 2001; 3:157–160.
16. Cho N, Kim HW, Lee HK, Jeon BJ, Sung SH. Biosci Biotechnol Biochem. 2016; 80:166–171.
17. Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH. Bioorg Chem. 2016; 66:97–101.
18. Matsuda H, Ishikado A, Nishida N, Ninomiya K, Fujiwara H, Kobayashi Y, Yoshikawa M. Bioorg Med Chem Lett. 1998; 8:2939–2944.
19. Lee M, Park JH, Min DS, Yoo H, Park JH, Kim YC, Sung SH. Biosci Biotechnol Biochem. 2012; 76:1616–1620.
20. Lee M, Sung SH. Pharmacogn Mag. 2016; 12:276–281.
21. Le CF, Kailaivasan TH, Chow SC, Abdullah Z, Ling SK, Fang CM. Int Immunopharmacol. 2017; 44:203–210.
22. Prachayasittikul S, Saraban P, Cherdtrakulkiat R, Ruchirawat S, Prachayasittikul V. EXCLI J. 2010; 9:1–10.
23. Kim JH, Byun JC, Bandi AKR, Hyun CG, Lee NH. J Med Plants Res. 2009; 3:914–920.
24. Kim MH, Nugroho A, Choi J, Park JH, Park HJ. Arch Pharm Res. 2011; 34:971–978.
25. Parmar VS, Vardhan A, Taneja P, Sinha R, Patnaik GK, Tripathi SC, Boll PM, Larsen S. J Chem Soc Perkin Trans 1. 1991; 11:2687–2690.
26. Ibrahim SR, Fouad MA, Abdel-Lateff A, Okino T, Mohamed GA. Nat Prod Res. 2014; 28:1765–1771.
27. Sunnerheim-Sjöberg K, Knutsson PG. J Chem Ecol. 1995; 21:1339–1348.
28. Ohta S, Koyama M, Aoki T, Suga T. Bull Chem Soc Jpn. 1985; 58:2423–2424.
29. Onoja E, Ndukwe IG. J Nat Prod Plant Resour. 2013; 3:57–60.
30. Awan ZI, Habib-ur-Rehman KAY, Minhas FA. IOSR J Appl Chem. 2013; 5:58–66.
31. Fuchino H, Konishi S, Satoh T, Yagi A, Saitsu K, Tatsumi T, Tanaka N. Chem Pharm Bull (Tokyo). 1996; 44:1033–1038.
32. Terazawa M, Koga T, Okuyama H, Miyake M. J Jap Wood Res Soc. 1973; 19:47–48.
33. Kim SH, Park JH, Kim TB, Lee HH, Lee KY, Kim YC, Sung SH. Bioorg Med Chem Lett. 2010; 20:2824–2827.
34. Liao CR, Kuo YH, Ho YL, Wang CY, Yang CS, Lin CW, Chang YS. Molecules. 2014; 19:9515–9534.
35. You YJ, Nam NH, Kim Y, Bae KH, Ahn BZ. Phytother Res. 2003; 17:341–344.
36. Rao SD, Rao BN, Devi PU, Rao AK. Orient J Chem. 2017; 33:173–180.
37. Jang GU, Choi SU, Lee KR. Yakhak Hoeji. 2005; 49:244–248.
38. Sidova V, Zoufaly P, Pokorny J, Dzubak P, Hajduch M, Popa I, Urban M. PLoS One. 2017; 12:e0171621.
39. Dinić J, Novaković M, Podolski-Renić A, Vajs V, Tešević V, Isaković A, Pešić M. Chem Biol Interact. 2016; 249:36–45.
40. Chokchaisiri R, Pimkaew P, Piyachaturawat P, Chalermglin R, Suksamrarn A. Rec Nat Prod. 2014; 8:46–50.
41. Tsao SM, Yin MC. J Agric Food Chem. 2015; 63:3196–3204.
42. Dong GZ, Jeong JH, Lee YI, Lee SY, Zhao HY, Jeon R, Lee HJ, Ryu JH. Arch Pharm Res. 2017; 40:509–517.
43. Tung NH, Kwon HJ, Kim JH, Ra JC, Ding Y, Kim JA, Kim YH. Bioorg Med Chem Lett. 2010; 20:1000–1003.
44. Ilic-Tomic T, Sokovic M, Vojnovic S, Ciric A, Veljic M, Nikodinovic-Runic J, Novakovic M. Planta Med. 2017; 83:117–125.
45. Park D, Kim HJ, Jung SY, Yook CS, Jin C, Lee YS. Chem Pharm Bull. 2010; 58:238–241.
46. Yadav D, Singh SC, Verma RK, Saxena K, Verma R, Murthy PK, Gupta MM. Phytomedicine. 2013; 20:124–132.
47. Novaković M, Novaković I, Cvetković M, Sladić D, Tešević V. Braz J Bot. 2015; 38:441–446.
48. Novaković M, Pešić M, Trifunović S, Vučković I, Todorović N, Podolski-Renić A, Dinić J, Stojković S, Tešević V, Vajs V, Milosavljević S. Phytochemistr. 2014; 97:46–54.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download