INTRODUCTION
MATERIALS AND METHODS
Development Dataset
Image Acquisition
Table 1
Standardized MRI Protocol in Institution A
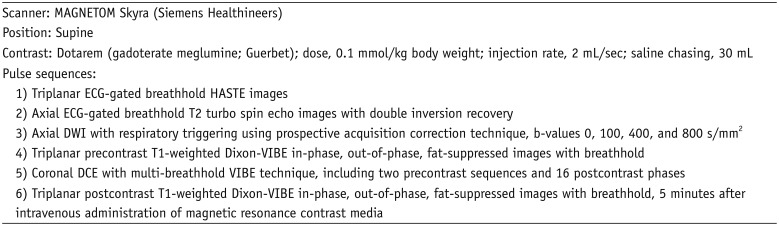
Image Analyses
Validation Dataset
Observer Tests
Statistical Analyses
RESULTS
Comparison between Cysts and Solid Masses in Development Dataset
 | Fig. 1Representative case from development dataset (cyst).Axial T2-weighted HASTE image (A), ADC map (B), pre- and post-contrast fat-suppressed T1-weighted images (C), and RER map (D) from 70-year-old male patient revealed 3.1 cm mass in anterior mediastinum (arrows). nT2, nADC, and RER values were 0.92, 1.50, and 5.3%, respectively. Pathologic diagnosis after surgical resection was thymic cyst. ADC = apparent diffusion coefficient, HASTE = half-Fourier-acquired single-shot turbo spin echo, nADC = normalized ADC, nT2 = normalized T2 signal intensity, RER = relative enhancement ratio
|
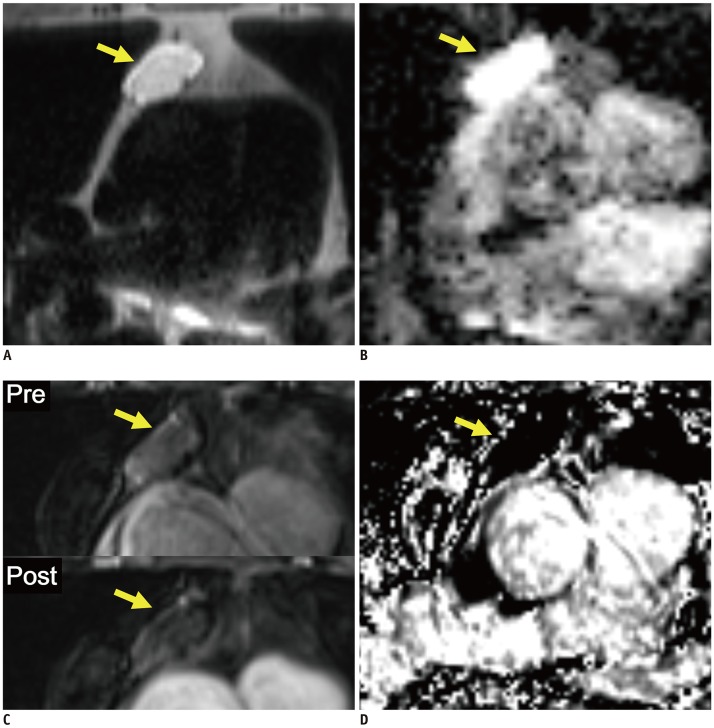
 | Fig. 2Representative case from development dataset (solid mass).Axial T2-weighted HASTE image (A), ADC map (B), pre- and post-contrast fat-suppressed T1-weighted images (C), and RER map (D) from 65-year-old male patient revealed 1.6 cm mass in anterior mediastinum (arrows). nT2, nADC, and RER values were 0.32, 0.76, and 59.6%, respectively. Pathologic diagnosis after surgical resection was thymoma, type B2.
|
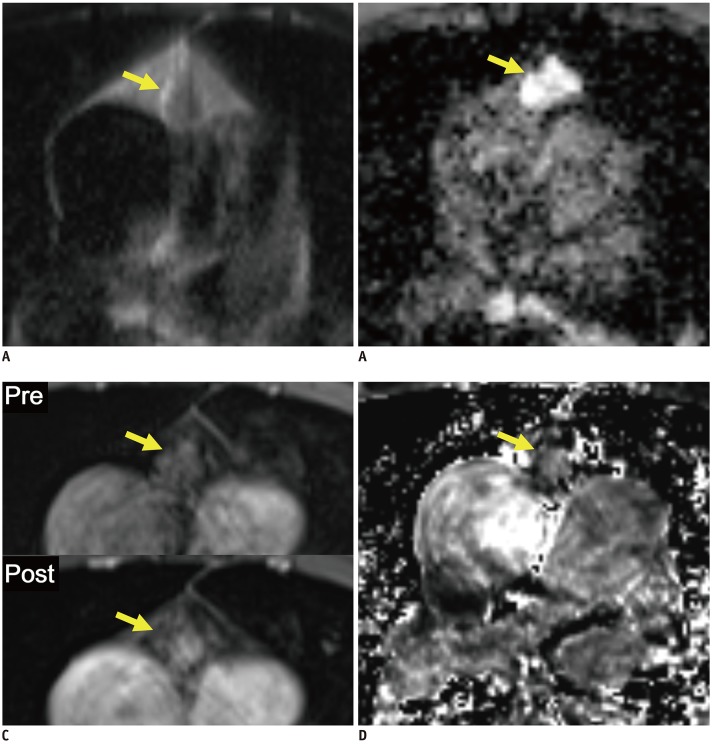
 | Fig. 3Boxplots and ROC curves for differentiation between cysts and solid masses in development dataset.Boxplots in left column show results of comparison of image variables between cysts and solid masses. nT2, nADC, and RER differed significantly between cysts and solid masses. Horizontal dashed lines indicate cutoff values developed in ROC analyses. ROC curves in right column show results of ROC analyses for differentiation between cysts and solid masses. Although nT2, and nADC appeared to be significant variables, RER showed perfect separation between cysts and solid masses. nT2, nT1, and nADC are fraction values without units. AUROC = area under receiver operating characteristic curve, ROC = receiver operating characteristic
|
Table 2
Comparison of Image Variables between Cysts and Solid Masses in Development Dataset
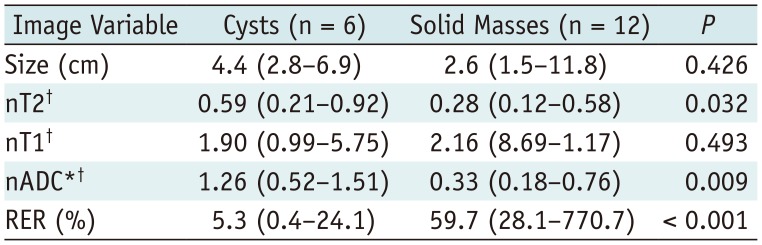
All data indicate median values with ranges in parentheses. *Missed in two cases (one cyst and one solid mass), †Fraction values without units. nADC = normalized apparent diffusion coefficient, nT1 = normalized T1 signal intensity, nT2 = normalized T2 signal intensity, RER = relative enhancement ratio
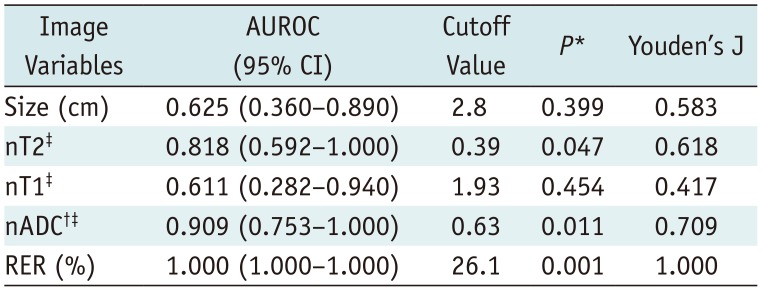
Development of Differentiation Criteria
Table 3
Results of ROC Analyses to Define Differentiation Criteria between Cysts and Solid Masses in Development Dataset
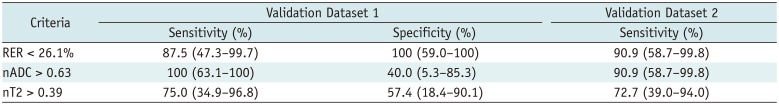
Validation of the Differentiation Criteria
Table 4
Comparison of Image Variables between Cysts and Solid Masses in Validation Dataset 1
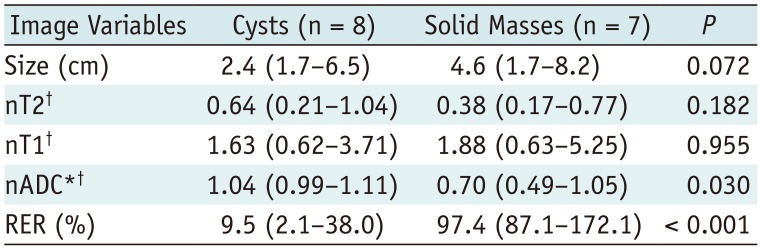
Table 5
Performances of Differentiation Criteria in Validation Datasets
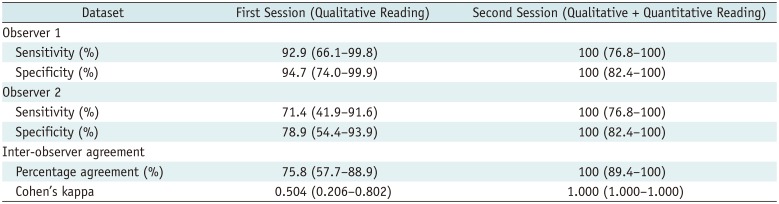
Observer Tests
Table 6
Results of Observer Test




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download