1. Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J Hepatol. 2012; 1:S25–S38.

2. Copelan A, Remer EM, Sands M, Nghiem H, Kapoor B. Diagnosis and management of Budd Chiari syndrome: an update. Cardiovasc Intervent Radiol. 2015; 38:1–12.

3. Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound elastography and MR elastography for assessing liver fibrosis: part 2, diagnostic performance, confounders, and future directions. AJR Am J Roentgenol. 2015; 205:33–40. PMID:
25905762.

4. Han KH, Yoon KT. New diagnostic method for liver fibrosis and cirrhosis. Intervirology. 2008; 51(Suppl 1):11–16. PMID:
18544943.

5. Srinivasa Babu A, Wells ML, Teytelboym OM, Mackey JE, Miller FH, Yeh BM, et al. Elastography in chronic liver disease: modalities, techniques, limitations, and future directions. Radiographics. 2016; 36:1987–2006. PMID:
27689833.

6. Yoon JH, Lee JM, Woo HS, Yu MH, Joo I, Lee ES, et al. Staging of hepatic fibrosis: comparison of magnetic resonance elastography and shear wave elastography in the same individuals. Korean J Radiol. 2013; 14:202–212. PMID:
23483022.

7. Mukund A, Pargewar SS, Desai SN, Rajesh S, Sarin SK. Changes in liver congestion in patients with Budd-Chiari Syndrome following endovascular interventions: assessment with transient elastography. J Vasc Interv Radiol. 2017; 28:683–687. PMID:
28153486.

8. Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008; 135:32–40. PMID:
18471441.

9. Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, et al. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007; 245:458–466. PMID:
17940304.

10. Trout AT, Sheridan RM, Serai SD, Xanthakos SA, Su W, Zhang B, et al. Diagnostic performance of MR elastography for liver fibrosis in children and young adults with a spectrum of liver diseases. Radiology. 2018; 287:824–832. PMID:
29470938.

11. Lee JE, Lee JM, Lee KB, Yoon JH, Shin CI, Han JK, et al. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography. Korean J Radiol. 2014; 15:210–217. PMID:
24643284.

12. Yoshimitsu K, Mitsufuji T, Shinagawa Y, Fujimitsu R, Morita A, Urakawa H, et al. MR elastography of the liver at 3.0 T in diagnosing liver fibrosis grades; preliminary clinical experience. Eur Radiol. 2016; 26:656–663. PMID:
26060066.

13. Rouvière O, Yin M, Dresner MA, Rossman PJ, Burgart LJ, Fidler JL, et al. MR elastography of the liver: preliminary results. Radiology. 2006; 240:440–448. PMID:
16864671.

14. Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014; 24:70–78. PMID:
23928932.

15. Tan CH, Venkatesh SK. Magnetic resonance elastography and other magnetic resonance imaging techniques in chronic liver disease: current status and future directions. Gut Liver. 2016; 10:672–686. PMID:
27563019.

16. Lee YJ, Lee JM, Lee JE, Lee KB, Lee ES, Yoon JH, et al. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2014; 39:326–331. PMID:
23589232.

17. Noone TC, Semelka RC, Siegelman ES, Balci NC, Hussain SM, Kim PN, et al. Budd-Chiari syndrome: spectrum of appearances of acute, subacute, and chronic disease with magnetic resonance imaging. J Magn Reson Imaging. 2000; 11:44–50. PMID:
10676619.

18. Cai SF, Gai YH, Liu QW. Computed tomography angiography manifestations of collateral circulations in Budd-Chiari syndrome. Exp Ther Med. 2015; 9:399–404. PMID:
25574205.

19. Cho OK, Koo JH, Kim YS, Rhim HC, Koh BH, Seo HS. Collateral pathways in Budd-Chiari syndrome: CT and venographic correlation. AJR Am J Roentgenol. 1996; 167:1163–1167. PMID:
8911174.

20. Wagner M, Corcuera-Solano I, Lo G, Esses S, Liao J, Besa C, et al. Technical failure of MR elastography examinations of the liver: experience from a large single-center study. Radiology. 2017; 284:401–412. PMID:
28045604.
21. Huang Q, Shen B, Zhang Q, Xu H, Zu M, Gu Y, et al. Comparison of long-term outcomes of endovascular management for membranous and segmental inferior vena cava obstruction in patients with primary Budd-Chiari Syndrome. Circ Cardiovasc Interv. 2016; 9:e003104. PMID:
26908849.

22. Lee BB, Villavicencio L, Kim YW, Do YS, Koh KC, Lim HK, et al. Primary Budd-Chiari syndrome: outcome of endovascular management for suprahepatic venous obstruction. J Vasc Surg. 2006; 43:101–108. PMID:
16414396.

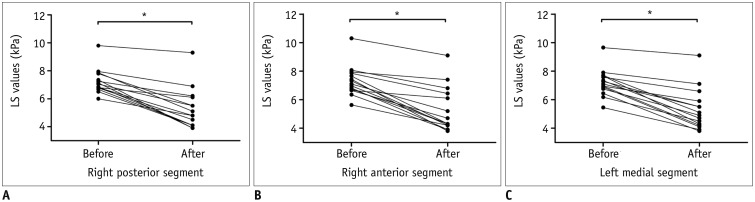
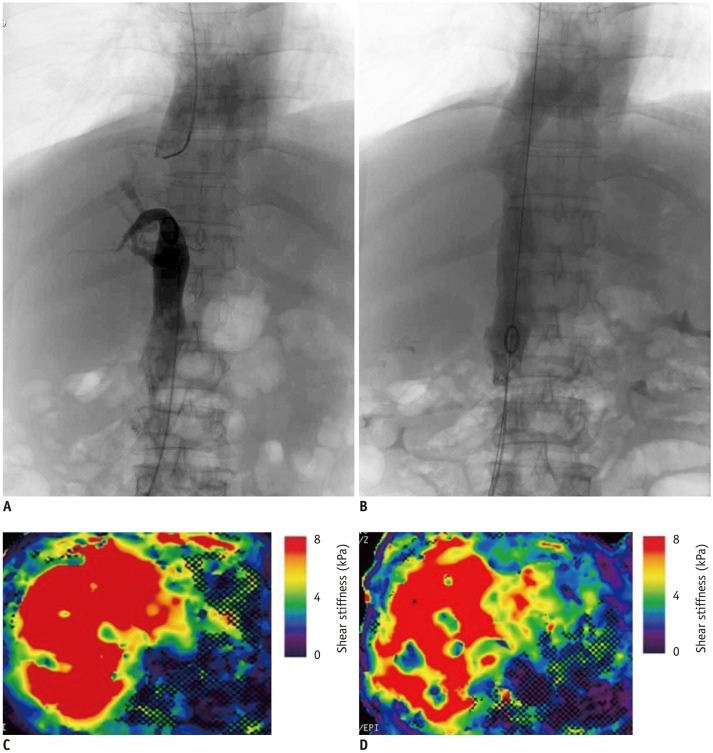
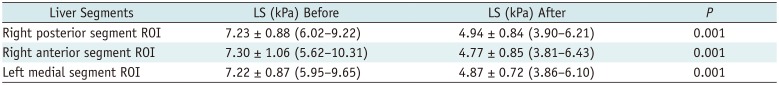
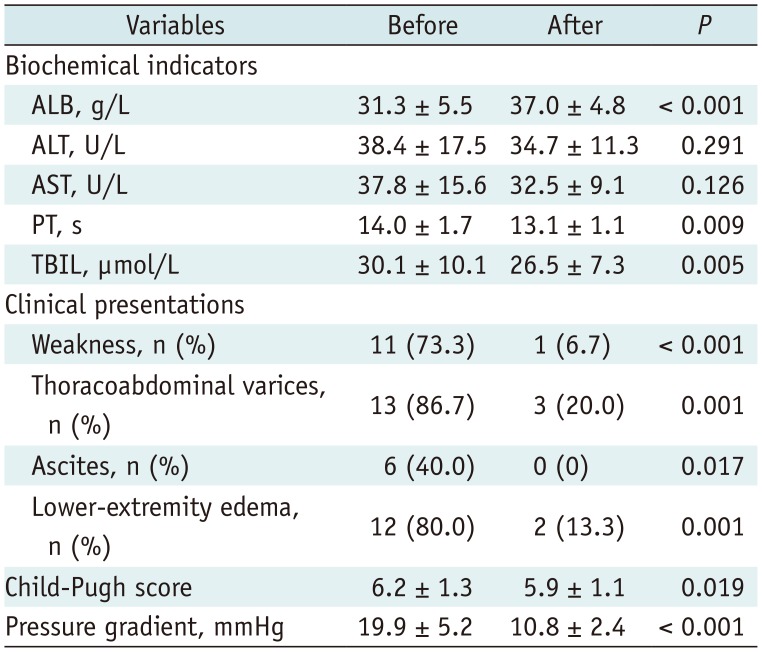




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download