1. Smith-Bindman R. Is computed tomography safe? N Engl J Med. 2010; 363:1–4. PMID:
20573919.

2. Leipsic J, Heilbron BG, Hague C. Iterative reconstruction for coronary CT angiography: finding its way. Int J Cardiovasc Imaging. 2012; 28:613–620. PMID:
21359835.

3. Silva AC, Lawder HJ, Hara A, Kujak J, Pavlicek W. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol. 2010; 194:191–199. PMID:
20028923.

4. Hou Y, Liu X, Xv S, Guo W, Guo Q. Comparisons of image quality and radiation dose between iterative reconstruction and filtered back projection reconstruction algorithms in 256-MDCT coronary angiography. AJR Am J Roentgenol. 2012; 199:588–594. PMID:
22915398.

5. Klink T, Obmann V, Heverhagen J, Stork A, Adam G, Begemann P. Reducing CT radiation dose with iterative reconstruction algorithms: the influence of scan and reconstruction parameters on image quality and CTDIvol. Eur J Radiol. 2014; 83:1645–1654. PMID:
25037931.

6. Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol. 2010; 195:649–654. PMID:
20729442.

7. Kröpil P, Bigdeli AH, Nagel HD, Antoch G, Cohnen M. Impact of increasing levels of advanced iterative reconstruction on image quality in low-dose cardiac CT angiography. Rofo. 2014; 186:567–575. PMID:
24458375.
8. Wang R, Schoepf UJ, Wu R, Reddy RP, Zhang C, Yu W, et al. Image quality and radiation dose of low dose coronary CT angiography in obese patients: sinogram affirmed iterative reconstruction versus filtered back projection. Eur J Radiol. 2012; 81:3141–3145. PMID:
22578834.

9. Yuki H, Utsunomiya D, Funama Y, Tokuyasu S, Namimoto T, Hirai T, et al. Value of knowledge-based iterative model reconstruction in low-kV 256-slice coronary CT angiography. J Cardiovasc Comput Tomogr. 2014; 8:115–123. PMID:
24661824.

10. Halpern EJ, Gingold EL, White H, Read K. Evaluation of coronary artery image quality with knowledge-based iterative model reconstruction. Acad Radiol. 2014; 21:805–811. PMID:
24809321.

11. Oda S, Weissman G, Vembar M, Weigold WG. Iterative model reconstruction: improved image quality of low-tube-voltage prospective ECG-gated coronary CT angiography images at 256-slice CT. Eur J Radiol. 2014; 83:1408–1415. PMID:
24873832.

12. Oda S, Utsunomiya D, Funama Y, Katahira K, Honda K, Tokuyasu S, et al. A knowledge-based iterative model reconstruction algorithm: can super-low-dose cardiac CT be applicable in clinical settings? Acad Radiol. 2014; 21:104–110. PMID:
24331272.
13. Zhang F, Yang L, Song X, Li YN, Jiang Y, Zhang XH, et al. Feasibility study of low tube voltage (80 kVp) coronary CT angiography combined with contrast medium reduction using iterative model reconstruction (IMR) on standard BMI patients. Br J Radiol. 2016; 89:20150766. PMID:
26607646.
14. Lee J, Park CH, Oh CS, Han K, Kim TH. Coronary computed tomographic angiography at 80 kVp and knowledge-based iterative model reconstruction is non-inferior to that at 100 kVp with iterative reconstruction. PLoS One. 2016; 11:e0163410. PMID:
27658197.

15. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006; 47(8 Suppl):C13–C18. PMID:
16631505.

16. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014; 8:342–358. PMID:
25301040.

17. Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N, et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017; 18:772–779. PMID:
27502292.
18. Schmid M, Pflederer T, Jang IK, Ropers D, Sei K, Daniel WG, et al. Relationship between degree of remodeling and CT attenuation of plaque in coronary atherosclerotic lesions: an in-vivo analysis by multi-detector computed tomography. Atherosclerosis. 2008; 197:457–464. PMID:
17727859.

19. Hu MQ, Li M, Liu ZY, Huang MP, Liu H, Liang CH. Image quality evaluation of iterative model reconstruction on low tube voltage (80 kVp) coronary CT angiography in an animal study. Acta Radiol. 2016; 57:170–177. PMID:
25657261.

20. Chang W, Lee JM, Lee K, Yoon JH, Yu MH, Han JK, et al. Assessment of a model-based, iterative reconstruction algorithm (MBIR) regarding image quality and dose reduction in liver computed tomography. Invest Radiol. 2013; 48:598–606. PMID:
23511193.

21. Hausleiter J, Martinoff S, Hadamitzky M, Martuscelli E, Pschierer I, Feuchtner GM, et al. Image quality and radiation exposure with a low tube voltage protocol for coronary CT angiography results of the PROTECTION II Trial. JACC Cardiovasc Imaging. 2010; 3:1113–1123. PMID:
21070998.
22. Feuchtner GM, Jodocy D, Klauser A, Haberfellner B, Aglan I, Spoeck A, et al. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: a comparative study. Eur J Radiol. 2010; 75:e51–e56. PMID:
19671491.

23. Park CH, Lee J, Oh C, Han KH, Kim TH. The feasibility of sub-millisievert coronary CT angiography with low tube voltage, prospective ECG gating, and a knowledge-based iterative model reconstruction algorithm. Int J Cardiovasc Imaging. 2015; 31(Suppl 2):197–203. PMID:
26521066.

24. André F, Fortner P, Vembar M, Mueller D, Stiller W, Buss SJ, et al. Improved image quality with simultaneously reduced radiation exposure: knowledge-based iterative model reconstruction algorithms for coronary CT angiography in a clinical setting. J Cardiovasc Comput Tomogr. 2017; 11:213–220. PMID:
28314613.

25. Iyama Y, Nakaura T, Yokoyama K, Kidoh M, Harada K, Oda S, et al. Low-contrast and low-radiation dose protocol in cardiac computed tomography: usefulness of low tube voltage and knowledge-based iterative model reconstruction algorithm. J Comput Assist Tomogr. 2016; 40:941–947. PMID:
27224224.
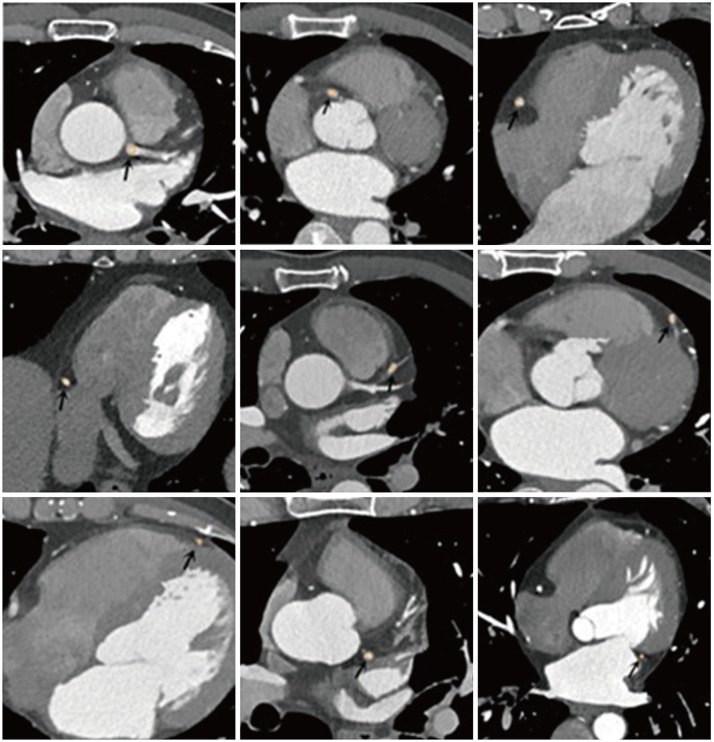
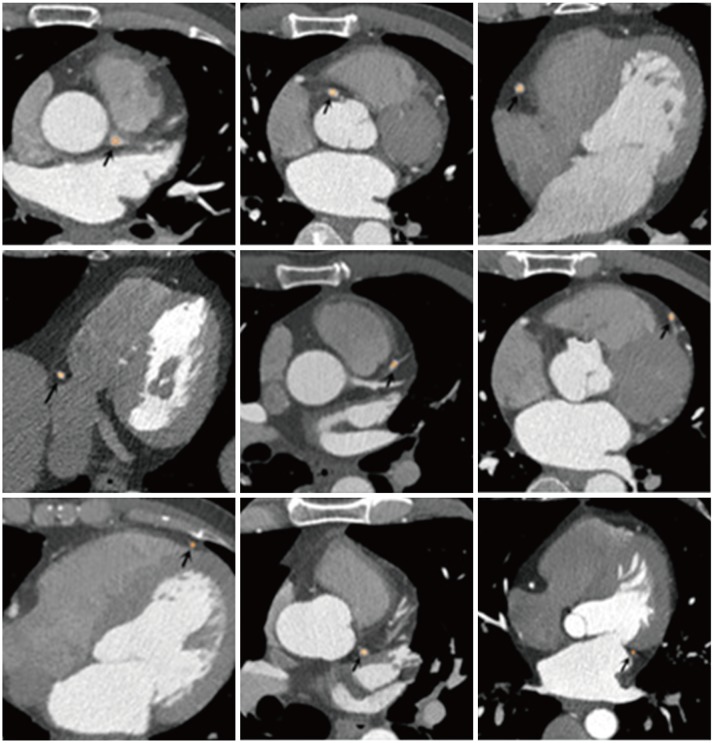
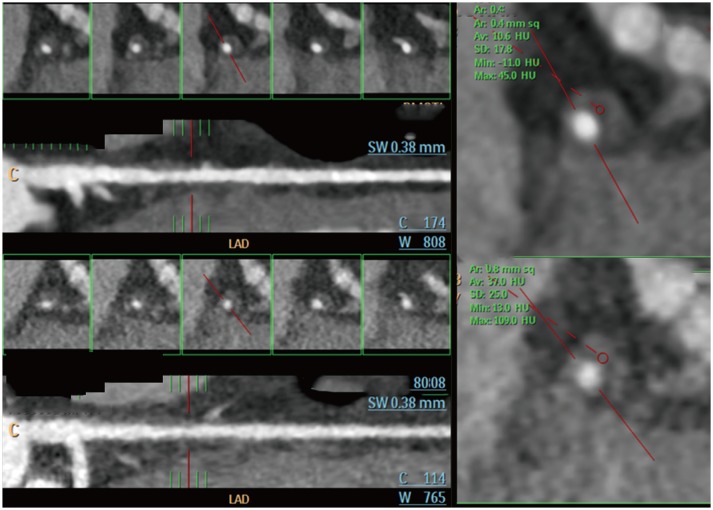
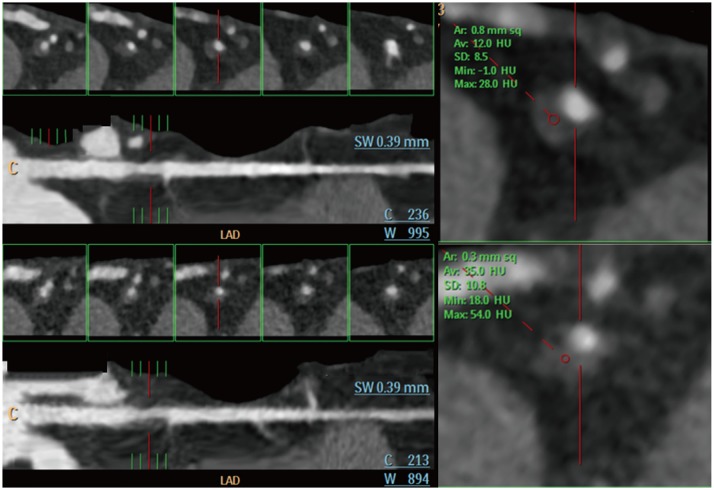




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download