Abstract
Background
Human Herpes Virus 6 (HHV6) is commonly associated with encephalitis following bone marrow transplantation. However, hippocampal atrophy and global hypometabolism are rare findings in HHV6 encephalitis.
Case Report
A 41-year-old right-handed woman with acute lymphoblastic leukemia presented with fever and mental changes 2 weeks after receiving a sibling bone marrow transplant. The patient's cerebrospinal fluid (CSF) was positive for HHV-6 deoxyribonucleic acid (DNA), but was negative for other viral DNA. Brain magnetic resonance imaging revealed atrophic changes in bilateral medial temporal lobes. Following 4 weeks of ganciclovir therapy, a CSF exam was negative for HHV-6 DNA and the patient's neurological symptoms partially improved. However, she was disoriented and had severe retrograde and anterograde amnesia. 18F-fluorodeoxyglucose-positron emission tomography indicated global hypometabolism in the medial temporal lobes and the fronto-parietal cortices.
Human Herpes Virus 6 (HHV6) is known to be commonly associated with encephalitis related to immunosuppression and transplantation, and to bone marrow transplantation (BMT) in particular.1 HHV-6 is known to be common in transplant recipients and lead to several clinical manifestations, such as encephalitis.1234 HHV 6 encephalitis in patients with BMT is known as latent infection. The first such case was reported in 1994.5 HHV6 encephalitis in patients receiving immunosuppressant drugs following BMT has various clinical manifestations, suggesting the involvement of the medial temporal lobe. These include confusion, seizure, and amnesia, which lead to so-called post-transplant acute limbic encephalitis.3 Neuroimaging findings in patients with HHV6 encephalitis correspond to those found in patients with acute limbic encephalitis.3678 However, cases of HHV6 encephalitis with hippocampal atrophy and glucose hypometabolism have very rarely been reported.
A 41-year-old right-handed woman with acute lymphoblastic leukemia (AML) presented with fever and mental change 2 weeks after BMT. She was alert, but had no awareness. She had no any other focal neurologic deficit. The treatment regimen following BMT consisted of 2 mg/kg cyclosporine, which was used to suppress immunity. The patient's past medical history was not significant, except for the presence of AML. She had no hypertension, diabetes mellitus, cardiac disease, chronic renal disease, or liver disease. She had no family history of a central nerve system (CNS) disease, hereditary disorder or developmental disorder. She was febrile, with a temperature of up to 39.3℃. Her vital signs were as follows: blood pressure was 115/70 mm Hg, heart rate was elevated up to 134 beats per minute, and respiratory rate was usually 30 breaths per minute. A complete blood count revealed anemia and thrombocytopenia due to AML, and mild leukocytosis. A blood chemistry study revealed the presence of mild hypocalcemia and elevated liver enzymes. C-reactive protein levels were slightly elevated. A chest X-ray study suggested the presence of interstitial pulmonary edema and subsegmental atelectasis and no evidence of pneumonia. Analysis of cerebro-spinal fluid (CSF) indicated 0 red blood cells (RBCs)/mm3, 0 white blood cells (WBCs)/mm3, a protein level of 59.5 mg/dL, and a glucose level of 181 mg/dL. Polymerase chain reaction (PCR) analysis of the CSF was positive for HHV-6 deoxyribonucleic acid (DNA), but was negative for other viral DNA, such as those of herpes simplex virus (HSV)-1, HSV-2, Epstein-Barr virus, cytomegalovirus (CMV), and varicella-zoster virus. There was no bacterial or fungal growth in the CSF and the CSF india ink test and the cryptococcal test were negative. Tests for CMV antigenemia and aspergillus antigen were negative in the serum. Brain magnetic resonance imaging (MRI) performed 5 days after symptom onset revealed atrophic changes in the bilateral medial temporal lobes. There was no abnormal high signal intensity or leptomeningeal enhancement, which suggested the presence of meningoencephalitis (Fig. 1). After 2 weeks of therapy with ganciclovir, a second CSF examination indicated 0 RBCs/mm3, 2 WBCs/mm3 (50% monocytes, 20% lymphocytes, and 1% neutrophils), a protein level of 92.6 mg/dL, and a glucose level of 118 mg/dL. The CSF was negative for HHV-6 DNA. After 4 weeks of anti-viral therapy, the neurological symptoms were partially improved. However, the patient was disoriented and complained of severe retrograde and anterograde amnesia. Eight weeks after BMT, her Mini-Mental State Examination score was 16; she was incorrect on the time and place orientation, serial 7, recall, interlocking pentagon, and language tests. She was even incorrect when asked if she had graduated from a graduate school. She could not remember the names of her parents, her current address, the name of the high school she graduated from, or recent events a few days old. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) performed 8 weeks after BMT indicated global hypometabolism in the bilateral medial temporal lobes and the fronto-parietal cortices (Fig. 2).
This is a rare and unusual case with atypical imaging findings on brain MRI and FDG-PET in a patient with HHV6 encephalitis. An MRI study performed in the acute stage revealed bilateral hippocampal atrophy. An FDG-PET study revealed global hypometabolism in the medial temporal lobes.
HHV6 encephalitis is not rare in immunocompromised patients with hematopoietic stem-cell transplantation.91011 After HHV6 was first isolated from patients with lymphoproliferative disorders and acquired immunodeficiency syndrome in 1986,12 it came to be known to be commonly associated with encephalitis related to transplantation.5 HHV6 encephalitis in patients with BMT is thought to be due to reactivation of HHV6 virus. HHV6 has been categorized into variants A and B.13 Primary HHV-6B infection is the cause of the common childhood illness roseola infantum,14 latent HHV-6B infection was well known the cause of HHV6 encephalitis in patients with BMT. Numerous such cases have been reported. Clinically, HHV6 encephalitis usually develops 2–6 weeks after BMT.310 Like other members of the herpesviridae family, it causes typical limbic encephalitis. Thus, symptoms such as mental change and amnesia are common and may provide diagnostic clues.31011
CSF PCR is the most definitive and essential test for the diagnosis of HHV6 encephalitis.6 HHV6 may be detectable in blood, as primary HHV6 infection during infancy results in generally mild, self-limited illnesses, and can be asymptomatic.1516 Therefore, the detection of HHV6 DNA in the CSF by PCR is considered to be substantive evidence of active CNS infection in patients with BMT.6 In this case, the patient showed symptoms of typical limbic encephalitis with fever within 2 weeks of BMT. PCR analysis was positive for HHV6 DNA in the CSF. Serological tests and PCR for other infectious agents were all negative. We were thus able to confirm our diagnosis.
In this case, the brain MRI study revealed chronic atrophic changes in the bilateral medial temporal lobes, rather than abnormal high signal intensity and leptomeningeal enhancement, which would suggest acute meningoencephalitis. Previous studies have shown that patients with HHV6 encephalitis after BMT have typical limbic encephalitis features in brain MRIs. These include high signal intensity in bilateral or unilateral medial temporal lobes, including in the hippocampus and the amygdala in T2/fluid-attenuated inversion recovery/diffusion weighted images.367 Some previous studies have reported HHV6 encephalitis cases where the hippocampal volume was notably reduced after treatment.317 In another study, MRI analysis in the late period showed severe atrophic changes in the affected bilateral medial temporal lobes.7 Usually, the atrophic change of HHV6 encephalitis were presented at the time about 3–4 weeks after symptom onset. In the previous studies, they had described "early volume loss" as the atrophic change after 3 weeks or 26 days after symptom onset.6717 Even considering this, the atrophy of our case was observed at an earlier time. The localization of high signal intensity or atrophy to the medial temporal lobes correlates well with neurologic deficits in patients with HHV6 encepahlitis.18 The clinical association between the medial temporal lobes and the dysfunction of memory formation is well-documented.192021 The severe retrograde and anterograde amnesia present in this case also correlate well with the patient's medial temporal lobe atrophy. It is unclear whether the medial temporal lobe atrophy was the result of the HHV6 encephalitis, associated finding, or incidental cooccurrence. However, considering the absence of significant previous medical history, her young age, and new symptom of severe retrograde and anterograde amnesia, the atrophic change on the patient's MRI point to HHV6 encephalitis and not to another preexistent disease. HHV6 encephalitis in immunocompromised patients is due to the reactivation of latent HHV6 infection. Thus, our imaging findings may be reflecting of the chronic indolent course of HHV6 encephalitis.22 Chronic latent HHV6 infection has been suggested to be present in other diseases, such as medial temporal epilepsy.232425
An analysis of FDG-PET in our patient revealed global hypometabolism, in the medial temporal lobes in particular. In previous studies, late-phase FDG-PET findings after anti-viral therapy indicated hypometabolism in areas with intense FDG uptake in the acute stage, such as in the bilateral hippocampi and the amygdala.817 Thus, the FDG-PET findings in this case correlate well with previous studies and with the patient's atrophy. Other possible considering cause of bilateral medial temporal atrophy and global hypometabolism in this case is the effect of immunosuppressive drug. The AML patient in this case received BMT after 6 months after diagnosis, any other medications were not administered to her during the 6 months. After BMT, cyclosporine and mycofenolate mofetil was administered as a prophylactic regimen of graft-versus-host disease. But, we thought the 2 weeks of administration was too short period to lead brain atrophy.
This is an unusual case of HHV6 encephalitis with medial temporal lobe atrophy, as seen on brain MRI. Our findings suggest a chronic indolent course for HHV6. HHV6 encephalitis cases are underestimated in the field of clinical neurology. With improved efficacy in diverse treatment methods employing transplantations in the hematology and oncology fields, neurologists should consider HHV-6 encephalitis as a differential diagnosis for acute to subacute encephalopathy in patients with transplantation.
Figures and Tables
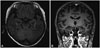 | Fig. 1Brain MR images in the patient. A: An axial fluid-attenuated inversion recovery image demonstrates an atrophic change in the bilateral medial temporal lobes without abnormal high signal intensity. B: A coronal contrast-enhanced T1-weighted image shows an atrophic change in the bilateral medial temporal lobes without leptomeningeal enhancement. MR: magnetic resonance. |
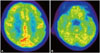 | Fig. 218F-Fludeoxyglucose-PET performed 8 weeks after transplantation shows global hypometabolism in the bilateral medial temporal lobes and the fronto-parietal cortices. A: Hypometabolism in fronto-parietal cortices. B: Hypometabolism in bilateral medial temporal lobes. PET: positron emission tomography. |
References
1. Yoshikawa T. Human herpesvirus 6 infection in hematopoietic stem cell transplant patients. Br J Haematol. 2004; 124:421–432.

2. Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace M, Armstrong RW, et al. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. 2005; 40:890–893.

3. Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007; 69:156–165.

4. Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, Kamble R. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007; 39:705–709.

5. Drobyski WR, Knox KK, Majewski D, Carrigan DR. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994; 330:1356–1360.

6. Gorniak RJ, Young GS, Wiese DE, Marty FM, Schwartz RB. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. AJNR Am J Neuroradiol. 2006; 27:887–891.

7. Noguchi T, Mihara F, Yoshiura T, Togao O, Atsumi K, Matsuura T, et al. MR imaging of human herpesvirus-6 encephalopathy after hematopoietic stem cell transplantation in adults. AJNR Am J Neuroradiol. 2006; 27:2191–2195.

8. Hubele F, Bilger K, Kremer S, Imperiale A, Lioure B, Namer IJ. Sequential FDG PET and MRI findings in a case of human herpes virus 6 limbic encephalitis. Clin Nucl Med. 2012; 37:716–717.

9. Ogata M, Fukuda T, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant. 2015; 50:1030–1036.

10. Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013; 57:671–681.

11. Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006; 193:68–79.

12. Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986; 234:596–601.

13. Dominguez G, Dambaugh TR, Stamey FR, Dewhurst S, Inoue N, Pellett PE. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J Virol. 1999; 73:8040–8052.

14. Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988; 1:1065–1067.
15. Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005; 352:768–776.
16. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005; 18:217–245.

17. Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001; 50:612–619.

18. Provenzale JM, van Landingham K, White LE. Clinical and imaging findings suggesting human herpesvirus 6 encephalitis. Pediatr Neurol. 2010; 42:32–39.

19. Milner B, Klein D. Loss of recent memory after bilateral hippocampal lesions: memory and memories-looking back and looking forward. J Neurol Neurosurg Psychiatry. 2016; 87:230.

20. Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000; 12:103–113.

21. Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20:11–21.

22. Yamane A, Mori T, Suzuki S, Mihara A, Yamazaki R, Aisa Y, et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transplant. 2007; 13:100–106.

23. Millichap JJ, Millichap JG. Role of HHV-6B Infection in Mesial Temporal Lobe Epilepsy. Pediatr Neurol Briefs. 2015; 29:40.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download