Abstract
Background and Purpose
During Vietnam War, many Korean soldiers were dispatched to fight in the war where they were exposed to Agent Orange. Until now, there exist only limited evidence on existence of association between exposure to Agent Orange and Parkinson's disease (PD). To elucidate the effects of Agent Orange exposure on PD, we compared the clinical characteristics and radiolabeled 18F-FP-CIT PET uptake between patients with Agent Orange exposure and patients with Agent Orange no-exposure.
Methods
We retrospectively evaluated 143 patients exposed to Agent Orange and 500 patients with no exposure to Agent Orange from our movement clinics database. The differences between clinical characteristics and pattern of 18F-FP-CIT PET uptake were investigated.
Results
Among Unified Parkinson's Disease Rating Scale III motor subscales, tremor at rest, rigidity, finger taps, and rapid alternating movement was significantly higher in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. The facial expression score was significantly lower in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. Compared to patients not exposed to Agent Orange, all basal ganglia areas (contra- and ipsilateral caudate nucleus, anterior putamen, and posterior putamen) showed a lower18F-FP-CIT uptake and higher asymmetry index of anterior and posterior putamen was found in patients exposed to Agent Orange. The caudate/putamen ratio was significantly lower in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange.
Conclusions
This study showed a different clinical profile and FP-CIT PET findings between patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. This finding suggests the possibility of different pathophysiology of PD in patients exposed to Agent Orange from idiopathic PD.
Agent Orange, which is an approximately 1:1 mixture of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyaceticacid (2,4,5-T), is one of the herbicides and defoliants used by the U.S. military during the Vietnam War. The 2,3,7,8-tetrachlorodibenzodioxin (TCDD), which is the product of 2,4,5-T contaminated with a dioxin, characteristically induces free radical oxygen generation and is one of the most toxic molecules responsible for causing various unexplained adverse health effects. The half-life of TCDD is approximately 8 years in humans and it is accumulated mainly in fatty tissues over time, so even exposure to small quantities may eventually lead to dangerous levels.
During the Vietnam War from 1965 to 1973, more than 300000 Korean troops were sent to fight in the war. Due to lack of appropriate information about Agent Orange at that time, many military troops are thought to have been exposed to this agent. More than 40 years after the war, there are many cases affecting Parkinson's disease (PD) among these veterans in Korea. Though, casual relationship between exposure to Agent Orange and PD is not certain yet, considering pathophysiological association free radical oxygen and PD, there may be a possibility of Agent Orange-induced or related PD. Unfortunately, there is a dearth of clinical evidence and studies on this relationship. To identify whether these patients are simply age-associated idiopathic PD (IPD) or secondary PD due to other causes such as Agent Orange is essential, because the cause might affect the diagnosis and treatment.
Movement Disorder Clinics of Veteran Health Service Medical Center is state-operated hospital clinics for veteran patients, and in case if PD is suspected, all the patients are examined by movement specialist and receive 18F-N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (18F-FP-CIT-PET) examination. Henceforth, it is conjectured that, we can build large-scaled 18F-FP-CIT PET database of PD by including patients with and without exposure to Agent Orange.
In this article, we compare the demographic, clinical characteristics, and imaging of 18F-FP-CIT PET between patients with and without exposure to Agent Orange.
A total of 762 patients with clinical evidence of PD were recruited between 2011 and 2015 from the Movement Disorders outpatient clinic of the Seoul Veteran Hospital. Among them, 643 patients (143 patients with exposure to Agent Orange and 500 patients with no exposure to Agent Orange) met step I and step II criteria according to the PD society brain bank.1 A brain magnetic resonance image scan (T1 and T2 weighted axial) was performed in all patients. The patients who had: 1) atypical PD syndromes; 2) history of stroke, cerebral tumor, traumatic brain injury, epilepsy, or psychiatric illness; 3) obvious medical complications; 4) farming as an occupation and history of pesticide exposure, were excluded from the present study.
The information about the veteran's unit was obtained from the Ministry of Defense's military records and veteran's self-reported survey. Using a perceived exposure index,2 the veterans were asked six questions regarding how they might have been exposed to Agent Orange in Vietnam and their responses were compared with official military records. Those who responded to these questions were classified into 1 of 4 groups with an associated perceived exposure index. To minimize the inclusion of false exposure group in the study, only the patients with 'moderate' and 'high' exposure were designated as "Agent Orange Exposure Group". The other patients, who met the above mentioned PD inclusion criteria and without any history of exposure to Agent Orange were designated as "Agent Orange No-exposure Group".
The Hoehn-Yahr staging (H-Y staging) and motor domain of the Unified Parkinson's Disease Rating Scale III, was used to evaluate severity of motor symptom. These scales were taken just before PET scanning when the antiparkinsonian drugs were stopped for at least 6 h. The clinical subgroups, tremor-dominant, and akinetic-rigid type were classified using the method designed by Lewis et al.3 If the tremor score was at least twice the non-tremor score, the patient was designated as tremor-dominant type. Vice versa, if the non-tremor score was at least twice the tremor score, the patient was designated as akinetic-rigid type. The remaining patients, in whom the tremor and non-tremor score differed by less than factor 2, were classified as mixed type. The patients' general cognitive state and severity of dementia were evaluated by means of the Mini-Mental State Examination. In any case, the patients were examined by a single neurologist who was unaware of the PET results after ceasing PD medication for at least 6 h. This study was approved by Institutional Review Board of Veteran Health Service Medical Center and meets the standards established by the Declaration of Helsinki.
All PD patients fasted for at least 12 h and discontinued all antiparkinsonian drugs for at least 6 h prior to PET studies. However, the drugs that may affect specific to nonspecific striatal binding, such as D-amphetamine, methylphenidate, benzatropine, buproprion, cocaine, mazindol, and phentermine, were restricted before the examination. Participants were administered 149 to 259 MBq of F-18 FP-CIT (3.7 MBq/kg) intravenously. Two sequential PET and computed tomography (CT) scans (dual timepoint) were acquired 90 and 210 minutes after the F-18 FP-CIT injection with the participant's eyes closed using Discovery STE (GE Healthcare, Milwaukee, WI, USA). The data were acquired in the 3-dimensional mode. CT scanning began at the vertex and progressed to the skull base (30 mAs; 140 kVp; slice, 3.75 mm), and PET imaging followed immediately over the same region with 15 min duration. The CT data were used for attenuation correction, and images were reconstructed using the standard ordered subset expectation maximization (OSEM: 2 iterations, 8 subsets) algorithm.
Additionally, image data from 10 normal subjects, who were examined by FP-CIT PET/CT, were used for estimating the imaginary matrix as described in this study. All the normal subjects (six males and four females; median age, 71 years; range 56–80 years) were examined by FP-CIT PET/CT voluntarily for reasons of private medical concerns.
Using the surface fitting method, the regions of interest (ROI) were identified and drawn on the caudate, putamen, and cerebellum in each hemisphere on MR images (1.5 T) realigned according to the PET image. The four adjacent slices where the striatum was best identified were used for ROI analysis. The putamen was divided into anterior and posterior halves along its longitudinal axis. The ROIs were then copied onto the PET image and the uptake of 18F-FP-CIT was calculated as a (region-cerebellum)/cerebellum ratio at 120 min after injection
Asymmetry index (AI) of putamen, caudate, and striatum was calculated according to the following formula: AI=[(ipsilateral-contralateral)/(ipsilateral+contralateral)]×100. The putamen caudate ratio (PCR) was calculated using the following formula PCR=putamen/caudate.4
First, the baseline demographic features, motor sub-symptoms, ROI of FP-CIT of the study participants with exposure to Agent Orange and no exposure to Agent Orange were assessed using Student's two-tailed t-tests. Second, the frequency according to H-Y stage and Parkinson subgroup difference were assessed using chi-square-tests. Statistical analyses were performed with the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
The study included 143 patients exposed to Agent Orange and 500 patients with no exposure to Agent Orange. Information regarding demographics of patients with exposure to Agent Orange versus no exposure to Agent Orange is documented in Table 1. The female prevalence was higher in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. The age, onset age, symptom duration, HY stage, and subtypes of PDs were not significantly different between the study subjects (Table 1).
Among UPDRS motor subscales, tremor at rest, rigidity, finger taps, and rapid alternating movement was significantly higher in patients with exposure to Agent Orange as compared to patients with no exposure to Agent Orange. The facial expression score was significantly lower in patients with exposure to Agent Orange as compared to patient with no exposure to Agent Orange (Table 2).
Among UPDRS hemispheric motor subscales, right resting tremor (arm and leg), right rigidity (arm and leg), right finger movement, and right hand movement were significantly higher in patients with exposure to Agent Orange as compared to patients with no exposure to Agent Orange (Table 3).
As compared to patients with no exposure to Agent Orange, patients with exposure to Agent Orange showed a lower FP-CIT uptake in all the basal ganglia areas (contra- and ipsilateral caudate nucleus, anterior putamen, and posterior putamen) and higher AI of anterior and posterior putamen (Table 4, Fig. 1). The caudate/putamen ratios were significantly lower in patients with exposure to Agent Orange as compared to patients with no exposure to Agent Orange (Table 4).
From 1962 to 1971, the US military used a considerable amount of Agent Orange during the Vietnam War to eliminate forest cover for North Vietnamese. Emerging toxicological evidence of phenoxy herbicides and TCDD from animal studies, some positive findings from epidemiologic studies, and various health problems in returning Vietnam veterans resulted in sustained controversy for toxicity of this agent. There exist differences in opinion concerning the neuronal adverse effects of TCDD. Some authors were skeptical about the facts that TCDD can cause any neurological damage.5 However, other authors did report few nervous system abnormalities in people exposed to TCDD.67 Moreover, regarding the association between Agent Orange and neurodegenerative disorders like PD, further studies are needed.
The Korean veterans exposed to Agent Orange during the Vietnam War are advancing in age and among them, many people are suffering from various neuro-degenerative disorders, like PD. To determine whether there is a scientifically relevant association between Agent Orange exposure and PD, appropriate epidemiological and prospective studies are of immense importance. However, to date, such studies are lacking. Moreover, in recent years, the number of this target population has decreased due to death associated with aging.
The pathophysiological hallmark of IPD is the profound deficit in brain dopamine level attributed to the loss of neurons of the nigrostriatal dopaminergic pathway,8 other causes of Parkinsonism show different pathological findings. The role of specific environmental risk factors (or toxicants) for PD development showed limited evidence. Several reviews of the literature have suggested the possible involvement of environmental chemicals in the etiology of PD. Systematic review and meta-analysis of the epidemiologic literature showed the relationship between PD and exposure to pesticides.9 The results suggest that exposure to pesticides, and to herbicides or insecticides in particular, increases the risk of PD. However, the relationship between exposure to Agent Orange and PD remains more controversial and largely unproven as compared to the data related to above-mentioned chemicals. Several studies have suggested 2,4-D and TCDD as main chemicals for neurotoxicity. A number of studies suggest that these chemicals exert neurologic effects (neurochemical and behavioral) in animal models, if exposure occurs during developmental stage or in cultured nerve cells.10
Generally, acute effects of toxicants may involve all the areas of the nervous system, whereas delayed effects are likely to produce more selective involvement of physiologically related systems of neurons like PD. Oxidative stress associated with the AhR-mediated induction of CYP1A1 isoenzyme11 and inflammation may cause selective dopaminergic neuronal damage in PD.12 In addition to the direct neuronal damage, indirect toxic effects to the nervous tissue may be induced by vascular impairment. There is strong evidence that the cerebral endothelium is the critical target of TCDD toxicity. Endothelial cell dysfunction can compromise the blood-brain barrier.13 Experimentally, exposure of rat cerebellar granule culture cells to 2,4-D led to an increase in concentrations of reactive oxygen species.14 However, numerous studies on neurotoxicity of 2,4-D have focused on its effects on the developing nervous system. These researches have often used high doses of 2,4-D that have resulted in adverse neuronal changes in the developing brain-both neurochemical (such as changes in D2 receptors, tyrosine hydroxylase, and dopamine beta-hydroxylase) and behavioral changes.15 Direct injection of 2,4-D into the rat brain resulted in the toxicity of basal ganglia.16 However, due to lack of clinical studies and PD model involving Agent Orange, above mentioned studies showed limited and non-conclusive evidence for association between PD and exposure to Agent Orange.
Among UPDRS motor subscales, patients with exposure to Agent Orange showed significantly lower facial expression, higher tremor at rest, rigidity, finger taps, and rapid alternating movement of hands as compared to patients with no exposure to Agent Orange. Interestingly, hemispheric UPDRS motor subscales and AI were higher in patients with Agent Orange exposure as compared to patients with no exposure to Agent Orange. The reason of high asymmetry in patients with Agent Orange exposure is not certain. Generally, a symmetric distribution of motor signs has been found to be associated with faster progression,17 a higher risk of balance disorder,17 more restrictions in activities of daily living,18 and with a higher risk of cognitive impairment.19 Previously reported studies suggested the possibility of better prognosis in patients with exposure to Agent orange, as compared to patients with no exposure to Agent Orange.
Degrees and patterns of FP-CIT uptake are significantly different in patients with exposure to Agent Orange as compared to patients with no exposure to Agent Orange. All the areas of FP-CIT uptake were significantly lower in patients with Agent Orange exposure as compared to their counterpart. Higher AI of putamen and lower caudate putamen ratio was found in patients with exposure to Agent Orange. These results suggested that patients with exposure to Agent Orange showed subtle differences in clinical and FP-CIT PET pattern as compared to patients with no exposure to Agent Orange. The FP-CIT PET imaging pattern suggested the possible severe damage to dopaminergic neuron in patients with exposure to Agent Orange as compared to patients with no exposure to Agent Orange. Why dopaminergic loss in putamen is severe in patients exposed to Agent Orange as compared to their counter-part is not certain. However, a great depletion in the level of dopaminergic markers in the putamen has also been reported in MPTP induced Parkinson model.20
We are unaware about why these differences occur despite similar demographic and H-Y staging. Possible hypothesis is that early toxicant exposure and slow neuronal damage by these chemicals may compensate for these structural damage and functional decline. For example, the long-term prognosis in early hemiparkinsonian patients is probably better than that in unilateral IPD due to slower disease progression, despite bilaterally reduced putamen dopaminergic terminal function.21
Therefore, it is hypothesized that slow neuronal damage and possible subsequent compensatory mechanisms might be responsible for extensive reduction in FP-CIT uptake in all striatal areas despite of similar H-Y stage. To clarify this hypothesis, the FP-CIT PET imaging study for patients with more mild or no symptoms of Agent Orange exposed PD may perhaps be necessary.
The present study has several limitations. First, the included study populations were mainly mild patients, and as a result, the study was biased for mild cases (mean H-Y stage 1.6 and 1.5 respectively). Second, though we meticulously recruited the patients exposed to Agent Orange based on self-reported survey and military records, due to compensation for Agent Orange related disease in Korea, the possibility of falsified study inclusion cannot be ruled out. We were not able to measure the actual dose of exposure to Agent Orange. Finally, the present study was a hospital based study, and so might not represent the real target populations.
In summary, our study showed different clinical findings and patterns of FP-CIT PET in patients with exposure to Agent Orange as compared to patients with no exposure to Agent Orange. The reasons behind existence of such differences in these patients are not certain, though possible hypothesis might be suggested through futuristic studies.
Figures and Tables
Fig. 1
Images of N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (FP-CIT PET) uptake at the level of striatum in patients with Hoehn-Yahr stage I Parkinson's disease. Patients with exposure to Agent Orange (A) display bilateral lower striatal uptake as compared with patients with no exposure to Agent Orange (B).
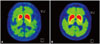
Table 1
Demographic characteristics and subtypes of PD between Agent Orange exposure and Agent Orange no-exposure groups (mean±standard deviation, number)

Acknowledgements
This study was supported by a VHS Medical Center Research Grant, Republic of Korea (grant number: VHSMC 15012).
References
1. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–184.

2. Decouflé P, Holmgreen P, Boyle CA, Stroup NE. Self-reported health status of Vietnam veterans in relation to perceived exposure to herbicides and combat. Am J Epidemiol. 1992; 135:312–323.

3. Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005; 76:343–348.

4. Isaias IU, Benti R, Cilia R, Canesi M, Marotta G, Gerundini P, et al. [123I]FP-CIT striatal binding in early Parkinson's disease patients with tremor vs. akinetic-rigid onset. Neuroreport. 2007; 18:1499–1502.

5. Zober A, Ott MG, Messerer P. Morbidity follow up study of BASF employees exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) after a 1953 chemical reactor incident. Occup Environ Med. 1994; 51:479–486.

6. Peper M, Klett M, Frentzel-Beyme R, Heller WD. Neuropsychological effects of chronic exposure to environmental dioxins and furans. Environ Res. 1993; 60:124–135.

7. Neuberger M, Rappe C, Bergek S, Cai H, Hansson M, Jäger R, et al. Persistent health effects of dioxin contamination in herbicide production. Environ Res. 1999; 81:206–214.

8. Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003; 39:889–909.
9. van der Mark M, Brouwer M, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect. 2012; 120:340–347.

10. Konjuh C, García G, López L, de Duffard AM, Brusco A, Duffard R. Neonatal hypomyelination by the herbicide 2,4-dichlorophenoxyacetic acid. Chemical and ultrastructural studies in rats. Toxicol Sci. 2008; 104:332–340.

11. Williamson MA, Gasiewicz TA, Opanashuk LA. Aryl hydrocarbon receptor expression and activity in cerebellar granule neuroblasts: implications for development and dioxin neurotoxicity. Toxicol Sci. 2005; 83:340–348.

12. Sarnico I, Boroni F, Benarese M, Sigala S, Lanzillotta A, Battistin L, et al. Activation of NF-kappaB p65/c-Rel dimer is associated with neuroprotection elicited by mGlu5 receptor agonists against MPP(+) toxicity in SK-N-SH cells. J Neural Transm (Vienna). 2008; 115:669–676.

13. Filbrandt CR, Wu Z, Zlokovic B, Opanashuk L, Gasiewicz TA. Presence and functional activity of the aryl hydrocarbon receptor in isolated murine cerebral vascular endothelial cells and astrocytes. Neurotoxicology. 2004; 25:605–616.

14. Bongiovanni B, Ferri A, Brusco A, Rassetto M, Lopez LM, Evangelista de Duffard AM, et al. Adverse effects of 2,4-dichlorophenoxyacetic acid on rat cerebellar granule cell cultures were attenuated by amphetamine. Neurotox Res. 2011; 19:544–555.

15. Bortolozzi AA, Evangelista De Duffard AM, Duffard RO, Antonelli MC. Effects of 2,4-dichlorophenoxyacetic acid exposure on dopamine D2-like receptors in rat brain. Neurotoxicol Teratol. 2004; 26:599–605.

16. Bortolozzi A, Evangelista de Duffard AM, Dajas F, Duffard R, Silveira R. Intracerebral administration of 2,4-diclorophenoxyacetic acid induces behavioral and neurochemical alterations in the rat brain. Neurotoxicology. 2001; 22:221–232.

17. Hely MA, Morris JG, Reid WG, O'Sullivan DJ, Williamson PM, Broe GA, et al. Age at onset: the major determinant of outcome in Parkinson's disease. Acta Neurol Scand. 1995; 92:455–463.

18. Gómez-Esteban JC, Tijero B, Ciordia R, Berganzo K, Somme J, Lezcano E, et al. Factors influencing the symmetry of Parkinson's disease symptoms. Clin Neurol Neurosurg. 2010; 112:302–305.

19. Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009; 73:1469–1477.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download