Abstract
Background and Purpose
Although the treatment efficacy of memantine in Parkinson's disease dementia (PDD) has been reported after several weeks of administration, the long-term effects on brain perfusion and clinical symptoms remain unclear. The current study aimed to follow-up PDD patients after 18 months of memantine treatment using 99mTc hexamethylpropylene amine oxime single photon emission computed tomography (SPECT).
Methods
A total of 15 patients with PDD and 11 healthy participants were recruited into this study and they were assessed with brain SPECT, Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), Global Deterioration Scale (GDS), and Neuropsychiatric Inventory (NPI). Differences in regional cerebral blood flow (rCBF) between the two groups were evaluated at baseline. After 18 months of memantine administration, changes in brain perfusion, severity of dementia, cognition, and neuropsychiatric disturbances were examined in the patients with PDD.
Results
The PDD group showed hypoperfusion in most of the cortical, subcortical, and cerebellar areas compared to healthy controls at baseline. At the follow-up, changes in rCBF, CDR (p=0.32), sum of box of CDR (p=0.49), MMSE (p=0.61), GDS (p=0.79), and NPI (p=0.23) were not significant in the PDD patients.
Although Parkinson's disease (PD) is primarily characterized by motor symptoms, its association with dementia has been increasingly reported in a number of epidemiological and pathophysiological studies.1 PD patients have a six-fold increased risk of developing dementia when compared with individuals without PD.2 The problems related to PD dementia (PDD) include higher care costs, increased disability and mortality, and reduced quality of life.345 Thus, cognitive decline and subsequent development of dementia have been widely considered as a major aspect of PD.1
For the treatment of PDD, cholinesterase inhibitors have been tested since patients with PDD showed substantial cholinergic deficits.6 Although rivastigmine improved cognitive function and behavioral symptoms, not all PDD patients responded to it and high rates of adverse events such as nausea, vomiting, and tremor were reported.7 On the other hand, impairment in glutamatergic activity was found in dementia with Lewy bodies.8 Based on this rationale, previous studies in PDD patients have investigated the efficacy of the N-methyl D-aspartate (NMDA) receptor antagonist memantine that alleviates the toxic effects of increased levels of the excitatory neurotransmitter glutamate, and have provided evidence of significant treatment advantages including improved attention and episodic recognition memory.91011 However, the follow-up period in these studies was 24 weeks and changes in brain structure or function were not assessed with in vivo neuroimaging. Our group recently published a single photon emission computed tomography (SPECT) study which showed that treatment of PDD patients with memantine for 12 months prevented deterioration of not only cognitive and neuropsychiatric symptoms but also brain perfusion.12
On the basis of this background, the current prospective study aimed to investigate the changes in brain perfusion, severity of dementia, cognitive performance, and neuropsychiatric symptoms in PDD patients after 18 months of memantine administration, using 99mTc hexamethylpropylene amine oxime (HMPAO) SPECT and neuropsychological assessment.
Patients with PDD who had no history of head trauma, epilepsy, stroke, and other neurological or psychiatric disorders were recruited at Incheon St. Mary's Hospital (Incheon, Korea). The onset of PD preceded the development of dementia by at least 12 months in all patients. Participants were excluded if they had remarkable fluctuations in cognitive functions; recurrent vivid hallucinations; any signs of atypical parkinsonism; delirium; amnestic disorders; or cerebrovascular lesions on magnetic resonance imaging. Patients who were taking psychotropic medications were also excluded. Healthy control participants without major medical conditions were matched for age and gender with the patient group.
The initial dose of memantine was 5 mg in the morning and it was gradually increased to 20 mg (10 mg in the morning and 10 mg in the evening) from week 4. This maintenance dose remained unchanged throughout the study period. Safety assessments included physical and neurological examinations, vital signs, electrocardiography, laboratory tests (blood chemical values, hematologic tests, and urinalysis), and adverse events.
Written informed consent was obtained from all participants and the study protocol was approved by the Research Ethics Committee.
Clinical assessments were conducted at baseline and follow-up. Assessment of the medical history and physical and neurological examinations were performed by board-certified neurologists. PDD was diagnosed according to the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria for PD, and Diagnostic and Statistical Manual of Mental Disorders-4th edition criteria for dementia.13 Fluorinated N-3-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (18F-FP-CIT PET) was also used for the diagnosis of PD.
The severity of PD symptom was evaluated with the Hoehn-Yahr Scale.14 The Clinical Dementia Rating (CDR)15 and Global Deterioration Scale (GDS)16 were used to determine the overall severity of dementia. Global cognitive function and neuropsychiatric disturbances were assessed with the Mini-Mental State Examination (MMSE)17 and Neuropsychiatric Inventory (NPI),18 respectively.
Brain SPECT scans were performed at baseline and follow-up. The participants were injected intravenously with 1110 MBq of HMPAO in a dark and quiet room, while lying supine with eyes open. After 40 minutes, images were acquired with a dual-head gamma camera (NM640, GE Healthcare, Milwaukee, WI, USA) equipped with a low-energy, fan-beam collimator. All images were attenuation corrected and reconstructed in a 128×128 matrix with a voxel size of 3.9×3.9×3.9 mm (field of view=240 mm) using filtered back projection.
Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) was used for image processing and statistical modeling. All images were spatially normalized to the SPM SPECT template (Montreal Neurological Institute, McGill University, Montreal, Canada) and resliced to a voxel size of 2×2×2 mm3. The binary brain mask in SPM8 was applied to remove extracerebral signal. The images were then smoothed with a 16 mm full-width half-maximum isotropic Gaussian kernel.
Two-sample t-test was used to investigate the differences in regional cerebral blood flow (rCBF) between the patient group and healthy controls at baseline. Perfusion scaling was performed using the reference cluster normalization that provides a substantial increase of statistical power in neurodegenerative diseases.19 In brief, a standard global mean normalized analysis was conducted to detect rCBF increases in the patient group using a threshold of t>2.0.20 The mean values of raw rCBF were extracted from each image using MarsBar toolbox version 0.44 (http://marsbar.sourceforge.net/) and used as a scaling factor for the detection of rCBF decreases in the patient group. The statistical threshold was set at family-wise error (FWE) corrected p<0.05 at both voxel and cluster levels. Voxel-wise paired t-test was performed to analyze the changes in rCBF between baseline and follow-up in the PDD group using the same procedure and statistical threshold described above.
Differences in age and gender distribution between PDD patients and healthy controls at baseline were assessed with independent t-test and chi-square test, respectively. Changes in MMSE, CDR, sum of box of CDR, GDS, and NPI scores between baseline and follow-up in the patient group were evaluated with paired t-test or Wilcoxon signed-rank test. A two-tailed p value of less than 0.05 was considered statistically significant. All analyses were conducted with Stata version 13.1 (StataCorp., College Station, TX, USA).
A total of 15 patients with PDD and 11 healthy participants were recruited into this study. Demographic and clinical characteristics of the participants are presented in Table 1. At baseline, the two groups did not differ in age (t=1.50, p=0.15) and gender ratio (χ2=0.001, p=0.97). The mean interval between baseline and follow-up was 1.5±0.9 years. In the patient group, changes in MMSE (z=-0.51, p=0.61), CDR (z=-1.00, p=0.32), sum of box of CDR (z=-0.69, p=0.49), GDS (t=0.27, p=0.79), and NPI (t=1.25, p=0.23) were not significant at follow-up.
Results from the SPM analysis at baseline are demonstrated in Fig. 1. Two clusters with decreased rCBF were found in the PDD group. The first cluster encompassed the cerebellum and most of the cortical and subcortical areas including the temporal occipital fusiform cortex, postcentral gyrus, and insula (peak voxel: t=8.55; voxel level FWE-corrected p<0.001; x, y, z coordinates=-28, -60, -12; cluster size=27786 voxels). Hypoperfusion in the left lingual gyrus was also found in the patient group (peak voxel: t=7.83; voxel-level FWE-corrected p<0.001; x, y, z coordinates=-10, -98, 0; cluster size=809 voxels). However, areas with increased rCBF were not detected. In addition, in the comparison between baseline and follow-up in the patient group, significant changes in rCBF were not found.
The current brain perfusion SPECT study investigated the changes of rCBF in PDD patients after administration of a NMDA receptor antagonist for approximately 18 months. We compared the differences in rCBF between the patient group and healthy controls at baseline, and then analyzed the changes in rCBF at follow-up in the PDD group. Changes in cognitive function, severity of dementia, neuropsychiatric symptoms were also examined.
At baseline, the patient group showed rCBF decreases in most of the cortical and subcortical regions and cerebellum when compared to healthy controls. These results were in agreement with a previous study in demented PD patients that revealed significant perfusion decrements in all cortical regions. 21 Moreover, extensive metabolic deficits in the frontal, temporal, parietal, and occipital lobes were found in PDD patients. 22 Although other reports have suggested rCBF reduction mostly in the parieto-occipital cortex,2324 the inconsistency may be due to different imaging modalities or analytic strategies. In particular, the reference cluster approach used in our SPM analysis is more advantageous than SPM's default global normalization in detecting hypoperfusion associated with neurodegenerative process.19 This technique allowed us to identify large areas of hypoperfusion in spite of the stringent statistical threshold.
The PDD group did not demonstrate significant changes in cognition, dementia severity, neuropsychiatric symptoms, and brain perfusion after treatment with memantine for 18 months. These findings extend the results of a previous 12-month trial. 12 Several longitudinal studies have demonstrated poor prognosis over time in PDD patients.1 However, our results indicated that memantine therapy may be efficacious in delaying the clinical deterioration of dementia-related symptoms including cognitive decline and neuropsychiatric disturbances. The possible neuroprotective mechanism of memantine has been suggested in a previous animal study. Memantine markedly increased the expression of brain-derived neurotrophic factor (BDNF) and its receptor trkB mRNAs in a widespread area of rat brain.25 Interestingly, reduced levels of neurotrophins including BDNF have been reported in the nigrostriatal dopaminergic system of PD patients2627 and in the hippocampus of Alzheimer's disease patients.2829 Furthermore, administration of exogenous BDNF promoted the survival or dopaminergic neurons in animal models of PD.3031
Potential limitations of this study include a small sample size and a lack of comparative group at follow-up. In addition, the diagnostic criteria for PDD in our study have not yet been validated. However, it is still challenging to distinguish between PDD and dementia with Lewy bodies based on clinical criteria. We attempted to reduce this bias by only including patients who met the two sets of diagnostic criteria while simultaneously showing a reduced dopamine uptake in the striatum on 18F-FP-CIT PET.
Despite these limitations, we found diffuse hypoperfusion in the cortical, subcortical, and cerebellar regions in the PDD patients using the recently developed count normalization technique. More importantly, the severity of dementia, cognitive performance, neuropsychiatric symptoms, and brain perfusion were not significantly changed after 18 months of memantine treatment. Taken together, our findings implicate that memantine may delay the progression of brain perfusion deficits and clinical symptoms of PDD in the long term. Further extended prospective studies with larger sample sizes over longer periods are warranted to confirm and validate our preliminary results.
Figures and Tables
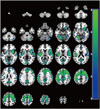 | Fig. 1Decreases in rCBF in the PDD group compared with the control group at baseline. Images are shown in neurological convention and color bar represents voxel-level t-values. PDD: Parkinson's disease dementia, rCBF: regional cerebral blood flow. |
Acknowledgements
This study was also supported by a grant of Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2015M3C7A1064832).
References
1. Docherty MJ, Burn DJ. Parkinson's disease dementia. Curr Neurol Neurosci Rep. 2010; 10:292–298.
2. Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology. 2001; 56:730–736.
3. Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000; 15:1112–1118.
4. Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006; 12:35–41.

5. Aarsland D, Brønnick K, Ehrt U, De Deyn PP, Tekin S, Emre M, et al. Neuropsychiatric symptoms in patients with Parkinson's disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007; 78:36–42.

6. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003; 60:1745–1748.

7. Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004; 351:2509–2518.

8. Dalfó E, Albasanz JL, Martin M, Ferrer I. Abnormal metabotropic glutamate receptor expression and signaling in the cerebral cortex in diffuse Lewy body disease is associated with irregular alpha-synuclein/phospholipase C (PLCbeta1) interactions. Brain Pathol. 2004; 14:388–398.

9. Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, et al. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009; 8:613–618.

10. Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A, et al. Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010; 9:969–977.

11. Wesnes KA, Aarsland D, Ballard C, Londos E. Memantine improves attention and episodic memory in Parkinson's disease dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry. 2015; 30:46–54.

12. Song IU, Chung SW, Chung YA. Efficacy of an NMDA receptor antagonist for Parkinson's disease dementia: a brain perfusion SPECT study. Int J Imaging Syst Technol. 2014; 21:326–331.

13. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association;2000.

15. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39:1159–1165.

16. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The Validity of the Korean Version of Global Deterioration Scale. J Korean Neurol Assoc. 2002; 20:612–617.

17. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.

18. Choi SH, Na DL, Kwon HM, Yoon SJ, Jeong JH, Ha CK. The Korean version of the neuropsychiatric inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000; 15:609–615.

19. Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz HG, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer's disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009; 44:43–50.

20. Borghammer P, Aanerud J, Gjedde A. Data-driven intensity normalization of PET group comparison studies is superior to global mean normalization. Neuroimage. 2009; 46:981–988.

21. Antonini A, De Notaris R, Benti R, De Gaspari D, Pezzoli G. Perfusion ECD/SPECT in the characterization of cognitive deficits in Parkinson's disease. Neurol Sci. 2001; 22:45–46.

22. Yong SW, Yoon JK, An YS, Lee PH. A comparison of cerebral glucose metabolism in Parkinson's disease, Parkinson's disease dementia and dementia with Lewy bodies. Eur J Neurol. 2007; 14:1357–1362.

23. Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O'Brien JT. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage. 2003; 20:1309–1319.

24. Le Heron CJ, Wright SL, Melzer TR, Myall DJ, MacAskill MR, Livingston L, et al. Comparing cerebral perfusion in Alzheimer's disease and Parkinson's disease dementia: an ASL-MRI study. J Cereb Blood Flow Metab. 2014; 34:964–970.

25. Marvanová M, Lakso M, Pirhonen J, Nawa H, Wong G, Castrén E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol Cell Neurosci. 2001; 18:247–258.

26. Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, et al. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp Neurol. 2000; 166:127–135.

27. Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000; (60):277–290.

28. Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991; 7:695–702.

29. Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000; 33:199–227.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download