Abstract
Background
Frontotemporal dementia (FTD) with motor neuron disease (MND) is a syndrome of progressive changes in behavior, language, muscle weakness and atrophy due to loss of function of neurons in the frontal and temporal lobes and in motor neurons. Etiology and pathogenesis of FTD with MND are still uncertain.
Case Report
A 71-year-old man presented with a 2-year history of progressive muscle weakness and cognitive deficits. We diagnosed this patient as FTD with MND by neurological examination, electromyography, brain imaging and neuro-psychological evaluation. We also confirmed antiphospholipid syndrome (APS) in this patient as a way to rule out secondary causes of MND.
Frontotemporal dementia (FTD) is a syndrome of progressive changes in behavior and language due to loss of function of neurons in the frontal and temporal lobes. Around 15% of patients with FTD meet formal clinical criteria for motor neuron disease (MND).1 Patients with FTD with MND may present with behavioral changes and/or language function declines seen in other subtypes of FTD.12 In this syndrome, these changes are accompanied by deterioration of motor neurons that manifests as weakness and atrophy in the muscles, fine muscle twitches and cramps and difficulty making fine movements.2 The cause of FTD with MND is unknown. Among many hypotheses, autoimmune mechanisms are considered as a possible etiology of the disease.34 FTD with MND in autoimmune pathology is very rare. Here we report about a patient who was diagnosed with FTD with MND and confirmed antiphospholipid syndrome (APS).
A 71-year-old man presented with a 2-year history of right upper extremity weakness, a 1-year history of left upper extremity weakness and a 1½-year history of weakness in both lower limbs. When this patient arrived at our hospital he was ambulating in a wheelchair and could stand for only a few seconds. He had other underlying diseases as well-epilepsy, angina pectoris and pulmonary thromboembolism (PTE). There was no family history of similar disease or exposure to any toxins or drugs. In the physical examination, weakness of upper limbs with Medical Research Council (MRC) grade 4 and weakness of lower limbs with MRC grade 3–4 were observed. Upper motor neuron signs were seen in the cervical region (Hoffman sign) and lower motor neuron signs were seen in bulbar (dysphagia, tongue atrophy and fasciculation) and lumbar regions (lower extremity atrophy and fasciculation). To confirm MND, electromyography (EMG) was performed and revealed normal neuronal conduction, but in needle EMGs there were frequent fasciculations and fibrillations in the upper and lower extremities (biceps, triceps, abductor pollicis brevis, vastus medialis, tibialis anterior muscles), the thoracic paraspinal muscles and bulbar muscles (Table 1). These findings were compatible with motor neuron involvement.
He also had memory impairment, decreased speech, inappropriate affect, apathy and irritability. He was a middle school graduate. He scored 16 on the Korean version of Mini-Mental State Examination. On the Seoul Neuropsychological Screening Battery, his verbal and visual memory functions showed impairment and he got particularly low scores on frontal executive function. Scores on stroop test color reading, semantic word fluency, and phonemic word fluency all fell below normal limits, suggestive of frontotemporal dysfunction (Table 2). In brain magnetic resonance imaging, cortical atrophy in both parietal and anterior temporal lobes was seen (Fig. 1). Fluorodeoxyglucose positron emission tomopraphy imaging demonstrated hypometabolism in the bilateral fronto-temporo-parietal cortex (Fig. 2). He was diagnosed with FTD with MND based on the neuropsychological and electrophysiological background.
To rule out secondary causes of the disease further laboratory tests were done. Lupus anticoagulant was positive and anti-cardiolipin Ab and anti-B2 glycoprotein 1 were negative. A follow-up lupus anticoagulant was done after three months and also showed a positive result. The patient was diagnosed with APS because he had a clinical episode of PTE and two laboratory lupus anticoagulant occasions at 12 weeks apart. He was treated as anticoagulant because he had APS and PTE.
FTD with MND is considered important because it may help to identify the pathophysiology of FTD, a highly heterogeneous disorder. Despite numerous pathological and genetic discoveries, much remains uncertain in FTD with MND.2 One of the most significant features of the disease is the substantial clinical heterogeneity and variability in disease prognosis. Around 15% of patients meet the criteria for both FTD and MND, the combination associated with a worse prognosis and reduction in survival time of around 1 year.56
In the previous studies, an inflammatory pathogenesis to neurodegenerative disease has long been hypothesized. Many neurodegenerative conditions are united by pathological protein misfolding and aggregation accompanied by neuronal loss and inflammatory markers around the site of pathological injury. Several studies of environmental risk factors in sporadic behavioral variant FTD found a significant association with head trauma and a close-to-significant association with thyroid disease.7 Furthermore, elevations in cerebrospinal fluid cytokines, notably TNF-α, have previously been demonstrated in FTD.8 An association between MND and autoimmune diseases also have been suggested previously. A familial study separately using index cases of amyotrophic lateral sclerosis (ALS) and multiple sclerosis reported increased association. Another hospital record-linkage study demonstrated an association of Behçet disease, ulcerative colitis, and Wegener granulomatosis in the offspring of patients with ALS.9 However, the basis of these observations in relation to shared pathogenesis remains unclear. Immunologic intervention for ALS, including bone marrow transplants, has so far not been effective, despite evidence linking inflammatory processes to pathogenesis in ALS.10
APS is a pathological status that arose from excess accumulation of blood clots by antiphospholipid antibodies (aPLs).11 The syndrome may occur as a primary condition or along with the autoimmune pathophysiology. The major clinical features associated with APS include recurrent thrombosis and pregnancy losses. APS can cause arterial or venous blood clots, in any organ system, or pregnancy-related complications. In APS patients, the most common venous event is deep vein thrombosis of the lower extremities, and the most common arterial event is stroke. Other common findings are low platelet count, heart valve disease, and livedo reticularis. There are also associations between APS and headaches, migraines, and oscillopsia.12 But the presence of aPLs in the blood of patients with neurodegenerative disease is very rare. The relationship between neurodegenerative disease and APS has been proposed in several studies. Higher levels of aPLs was found in patients with dementia than in controls13 and a significant association between anticardiolipin and both vascular dementia and Alzheimer's disease was noted.14 This has also been demonstrated in experimental studies. An animal study was performed with BALB/c mice using a staircase test and a T maze alternation test as cognitive assessment tools. Mice immunized with anti-β2 glycoprotein I antibodies developed a higher degree of cognitive abnormalities than those that had not been immunized.15 In another study, the importance of TANK binding kinase-1 (TBK1), a multimeric kinase that modulates inflammation and autophagy, was suggested. In human health, that had been highlighted for the first time by the recent discoveries of mutations in TBK1 that underlie ALS and FTD.16 Until now, there are several studies with evidence of association between MND and autoimmune disease, but further research is needed.
There are limitations to this case. First, it is still unclear whether the relationship between FTD with MND and APS was causal or simply coincidence. To clarify that unknown further search for similar cases is needed. Second, we could not identify the significant inflammatory marker which suggested neurodegenerative pathology in serum or cerebrospinal fluid. Among several hypotheses which explain the etiology of FTD with MND, autoimmune mechanism is one. To the best of our knowledge, this is the first case of FTD with MND in APS in Korea. In this case, APS may induce its effect immunologically or by thrombosis of small arterioles and venules, microinfarcts of neuronal cells and resultant development of clinical manifestations. These hypotheses lead us to elucidate the autoimmune pathogenesis and provide an explanation for FTD with MND. Further evaluation and evidence in the pathophysiology of the disease is needed.
Figures and Tables
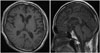 | Fig. 1Axial & sagittal T1-weighted brain MR images of the patient. Cortical atrophy was seen in both parietal and anterior temporal lobe. |
References
1. Rodríguez de Rivera FJ, Rambold HA, Díez-Tejedor E. Assessment of cognitive impairment in amyotrophic lateral sclerosis. J Neurol Sci. 2014; 337:1–2.

2. Burrell JR, Halliday GM, Kril JJ, Ittner LM, Götz J, Kiernan MC, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016; 388:919–931.

3. Miller ZA, Rankin KP, Graff-Radford NR, Takada LT, Sturm VE, Cleveland CM, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry. 2013; 84:956–962.

4. Niebroj-Dobosz I, Dziewulska D, Janik P. Auto-antibodies against proteins of spinal cord cells in cerebrospinal fluid of patients with amyotrophic lateral sclerosis (ALS). Folia Neuropathol. 2006; 44:191–196.

5. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007; 6:994–1003.

6. Talbot K, Ansorge O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Hum Mol Genet. 2006; 15:R182–R187.

7. Rosso SM, Landweer EJ, Houterman M, Donker Kaat L, van Duijn CM, van Swieten JC. Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case-control study. J Neurol Neurosurg Psychiatry. 2003; 74:1574–1576.

8. Sjögren M, Folkesson S, Blennow K, Tarkowski E. Increased intrathecal inflammatory activity in frontotemporal dementia: pathophysiological implications. J Neurol Neurosurg Psychiatry. 2004; 75:1107–1111.

9. Hemminki K, Li X, Sundquist J, Sundquist K. Familial risks for amyotrophic lateral sclerosis and autoimmune diseases. Neurogenetics. 2009; 10:111–116.

10. Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013; 136(Pt 2):471–482.

11. Atanassova PA, Dimitrov BD. Neurological and systemic disorders in antiphospholipid syndrome: novel constellations on a genetic basis. Clin Neurol Neurosurg. 2006; 108:814.

12. Turner MR, Goldacre R, Ramagopalan S, Talbot K, Goldacre MJ. Autoimmune disease preceding amyotrophic lateral sclerosis: an epidemiologic study. Neurology. 2013; 81:1222–1225.

13. Mosek A, Yust I, Treves TA, Vardinon N, Korczyn AD, Chapman J. Dementia and antiphospholipid antibodies. Dement Geriatr Cogn Disord. 2000; 11:36–38.

14. Juby A, Davis P, Genge T, McElhaney J. Anticardiolipin antibodies in two elderly subpopulations. Lupus. 1995; 4:482–485.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download