Abstract
Background and Purpose
This study was designed to evaluate the effect on sleep of rivastigmine transdermal patch in patients with probable Alzheimer's disease (AD).
Methods
Patients with probable AD underwent a sleep questionnaire, overnight polysomnography and neuropsychological tests before and after rivastigmine transdermal patch treatment. We analyzed the data from enrolled patients with AD.
Sleep disturbance in patients with probable Alzheimer's disease (AD) are frequent and disabling features, affecting approximately 25–60% of all patients. The most common sleep related complaints are insomnia, sleep fragmentation and excessive daytime sleepiness, frequently accompanied by sleep apnea in patients with probable AD.1 Change in sleep precedes the onset of cognitive symptoms in patients with AD, and a strong association exists between disrupted sleep and the development of AD.2 Cholinergic neuronal loss of Meynert nucleus in the basal forebrain is a pathophysiological hallmark of AD, and cholinergic activity in the central nervous system influences upper airway opening via the central and peripheral mechanism. Decreased thalamic pontine cholinergic projections may affect the respiratory drive, leading to both central and obstructive apnea in neurodegenerative diseases, including AD.34 The primary pharmacological treatments approved for AD are central acting cholinesterase inhibitors, and rivastigmine transdermal patches are frequently used in mild to moderate AD. The aim of this study was to evaluate the effects of rivastigmine transdermal patches on sleep architectures and sleep apnea in patients with probable AD.
Nineteen patients with probable mild to moderate Alzheimer's disease were consecutively recruited from the neurocognitive and dementia center at Myongji hospital. Patients who met the criteria for probable AD, established by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association,5 were included in the present study. They ranged from 50 to 90 years of age, and had a Korean Mini-Mental State Examination (K-MMSE)6 score of 10 to 20. The brain computed tomography or magnetic resonance imaging showed no clinical evidence of other diseases (i.e., normal pressure hydrocephalus, brain tumor, cerebrovascular disease) capable of producing a dementia syndrome. They had reliable caregivers who met the patient at least twice a week, and was sufficiently familiar with the patient to provide the investigator accurate information. We enrolled those patients with dementia, where the reliable caregivers observed and reported snoring and stopping of breathing during the night. The exclusion criteria included any primary neurodegenerative or psychiatric disorders other than AD (i.e., Parkinson's disease, schizophrenia, or major depressive disorder); clinically significant laboratory abnormalities (such as an abnormal thyroid function test, abnormally low levels of vitamin B12 or folate, or positive Venereal Disease Research laboratory test); any history of drug or alcohol addiction; any severe or unstable medical disease; any hearing or visual impairment that could disturb the efficient evaluation of the patient; any active skin lesion; a history of allergy to topical products containing any of the constituents of the patches; and an involvement in other clinical trials or treated by any an experimental drug within the past three months. Participants should not be taking any acetylcholinesterase inhibitors (ChEIs) or other anti-dementia drugs for at least four weeks prior to the baseline visit. Subjects were able to visit an outpatient clinic and to perform sleep study and cognitive function tests with walkers, canes or wheelchairs accompanied by their caregivers. The study was conducted in accordance with the Declaration of Helsinki and good clinical reporting practices. The study protocol and informed consent form were reviewed and approved by the institutional review board before commencement of the study. Prior to participation in the study, both the patients and their legal guardians or representatives gave their written informed consent to participate.
Enrolled patients took part in a sleep questionnaire to evaluate their sleep problems and sleep environments. They were also rated on the Epworth Sleepiness Scale (ESS)7 and the Stanford Sleepiness Scale (SSS)8 to measure daytime sleepiness, as well as the Beck Depression Inventory (BDI)9 to evaluate depressive moods. After completing the questionnaire, the subjects underwent two overnight polysomnographies (PSG), before and after rivastigmine transdermal patch application. PSG recorded the electroencephalography (EEG), electrooculography (EOG), chin electromyography (EMG), leg EMG, electrocardiography, and microphone for recording the sounds of snoring, plethysmography for evaluating respiratory airflow and effort, and oximetry for checking arterial oxygen saturation. PSG was performed overnight in a sleep laboratory with a sleep technologist in attendance. The scoring and staging followed the American Academy of Sleep Medicine clinical guidelines.10 Hypopnea was defined ≥50% airflow reduction and ≥3% desaturation or arousal. Sleep stages were based on 3 sources of data coming from 7 channels: EEG, EOG, and chin EMG. Sleep architecture considered the following variables: proportion of non-rapid eye movement (REM) sleep period; N1, N2, N3, and REM sleep periods from PSG data. Breathing event during sleep was evaluated as the Apnea Hypopnea Index (AHI) and Respiratory Disturbance Index (RDI), which included AHI and respiratory event related arousal. Sleep disordered breathing (SDB) was defined by AHI or RDI of 5 or higher, in association with excessive daytime somnolence. Other variables, including periodic limb movement during sleep (PLMS), total sleep time (TST), total wake time (TWT), wake time after sleep onset (WASO) and sleep onset latency (SL), and sleep efficiency (SE), were calculated from data of overnight PSG.
The K-MMSE was administrated at the screening, baseline and at the end of the study. The clinical dementia rating (CDR)11 and Korean version of AD assessment scale-cognitive (ADAS-cog) subscale12 were collected at the baseline and at end of the study. All of the above neuropsychological tests were performed with the same clinical psychologist for the entire period of the clinical trial.
Sleep variables, such as the proportion of N1, N2, N3, and REM, TST, TWT, WASO, SL, SE, RDI, AHI, PLMS index from overnight PSG and ESS, SSS, BDI from the sleep questionnaire and K-MMSE, CDR ADAS-cog score were compared with baseline and at 12 weeks after rivastigmine patches were applied. Comparative analysis between the data from the baseline study and the data from follow up studies for all enrolled patients with AD were analyzed by the chi-square test to compare categorical variables, and the paired t-test to compare continuous variables, using SPSS v 17.0 software (SPSS for Windows, SPSS Inc., Chicago, IL, USA).
Nineteen patients with probable AD with SDB were initially recruited in this study. Sleep and neurocognitive studies were performed at the baseline level for these nineteen patients with AD. After the baseline study, all enrolled patients were administrated 5 cm2 rivastigmine patch per day for 4 weeks. In the absence of any adverse events related with the patch, patients were treated to 10 cm2 rivastigmine patch for three months. However, five patients reported skin eruptions or erythema and itching at the site of application during the run-in period of four weeks. They were excluded from this study because of skin problems attributed to rivastigmine transdermal patch. Finally, fourteen patients achieved a target patch size of 10 cm2 rivastigmine patch from the initial patch application (Fig. 1). After 12 weeks from baseline sleep and neurocognitive studies, a follow up sleep and neurocognitive study was performed in these fourteen patients. They were 68.4±4.3 [mean±standard deviation(SD)] years old, and included five males and nine females. Their body mass index was 23.8±2.9 (mean±SD) kg/m2. Education status of enrolled patients was 4.02±3.04 (mean±SD) years, and K-MMSE was 19±3.0 (mean±SD) at the baseline level. ADAS-cog was 31±10.1 (mean±SD) at baseline level. All these patients did not consume coffee or caffeine containing tea. Three patients (two males, one female) reported initiation insomnia as well as SDB. One of these patients was on antihypertensive drug for 10 years. The other patients had no vascular risk factors such as diabetes, hyperlipidemia and hypertension, stroke or cardiac problems.
TST, TWT and proportion of N1, N2, N3 and REM sleep showed no statistical difference between the baseline and follow up values after rivastigmine patch application. The WASO, SL, SE, and PLMS index also did not show any difference from the baseline levels after treatment. However, RDI and AHI (28.4±14.4 and 24.76±28.2, respectively) markedly decreased after the rivastigmine patch treatment (p<0.05) compared to RDI and AHI at baseline level (45.3±18.4 and 41± 12.4, respectively). ESS and SSS from the sleep questionnaire showed no differences between baseline and after treatment. The sleep study findings are summarized in Table 1. The K-MMSE and CDR were 19±3.0 and 1.2±0.4 (mean±SD) at the baseline level, and 18±3.0 and 1.3±0.66 (mean±SD) after rivastigmine patch application, respectively. ADAS-cog scores were 31±10.1 (mean±SD) at baseline and 22.5±5.9 (mean± SD) after treatment. However, there was no statistical significance of cognition and in the functional state before and after rivastigmine transdermal patch treatment. These results are outlined in Table 1.
Several studies support the association between sleep apnea and AD.23 SDB reported a high prevalence (33% to 60%) of patients with AD, and is considered as an important non-cognitive symptom contributing to three clinical courses of AD.2 SDB is often reported to have an association with agitation or day time somnolence, other than cardiovascular risk, in patients with AD.1314 Therefore, treatment of SDB is considered to be a significant issue in the management of patients with AD. Ancoli-Israel et al.15 reported that continuous positive airway pressure (CPAP) reduced daytime somnolence in patients with mild to moderate AD with SDB. Cooke et al.16 reported that the long-term CPAP treatment for patients with AD and SDB may result in improvements of sleep and mood, as well as a slowing cognitive deterioration. Especially for patients with dementia, it is difficult to maintain CPAP therapy because most of the cognitive behavioral problems are aggravated during night time sleep. In contrast to previous studies on physical and surgical treatment for SDB, there is a dearth of effective pharmacological approaches.17 Very few studies have examined the effects of cholinergic treatment on sleep in patients with AD. A study on the effects of tacrine on REM sleep was not conclusive, probably since doses higher than 100 mg per day could not be administered due to hepatotoxicity.18 PSG data by Moraes et al.19 reported that donepezil inhibits acetylcholinesterase improved obstructive sleep apnea and increased REM sleep density and periods in patients with AD. No evidence of worsening sleep or nightmares were found in the above study. Suckys-Claudino et al.20 showed that donepezil treatment improved RDI, oxygen desaturation, and sleepiness. These studies with donepezil support the conceptual opinion that cholinergic transmission might influence breathing regulation in patients with AD/SDB. Meanwhile, rivastigmine transdermal patches are another form of acetylcholinesterase and butyrylcholinesterase inhibitors, which are currently approved for the treatment of patients with probable AD.21 A transdermal form of rivastigmine has several advantages, including continuous delivery with reduced fluctuation of plasma drug levels, improved tolerability, easy administration of optimal doses, and preference for caregivers.222324 We expect and focus the stable cholinergic properties of rivastigmine patches be tolerated by our enrolled subjects; however, adverse events, such as skin rash or itching sensation, developed in a few patients after rivastigmine patch application. The present study demonstrated that rivastigmine patch treatment has the potential to reduce RDI and improve sleep apnea or hypopnea, but did not changed other sleep parameters, namely sleep latency, SE, WASO or REM sleep density and periods. Another finding is that improved RDI of patients with AD on rivastigmine patch treatment was not statistically significant when correlated to cognitive function measured by ADAS-cog. To the best of our knowledge, this is the first PSG study on the effect of rivastigmine transdermal patches in patients with probable AD. Until now, most cholinergic drugs mainly increase the REM density and decrease the latency of REM onset. However, several studies have shown contradictory results, depending on the subjects or drugs enrolled in each study.2526 In particular, a recent study of rivastigmine in elderly persons significantly reduced the REM latency, while there was no effect on REM sleep proportion.27 In our study, the rivastigmine patch treatment did not affect the proportion of REM sleep or latency to REM onset, and REM density. We suggest that a large sample longitudinal study would be appropriate to estimate the effect of rivastigmine during REM sleep. In degenerative conditions such as dementia, the decreased thalamo-pontine cholinergic projections may affect the respiratory drive, leading to both central and obstructive sleep apnea.328 Based on this mechanism, increasing the cholinergic tone by ChEIs in thalamopontine cholinergic projections could improve SDB. We consider that the cholinergic action of rivastigmine transdermal patch is the main mechanism for improving SDB. In contrast, enhancing REM sleep of ChEIs might affect sleep apnea in a negative way, in spite of its positive effect to impaired cognitive functions. Such conflicting effects make it difficult to understand the actual mechanisms. A possible explanation is that rivastigmine increases the central cholinergic transmission in areas related to different tasks, including cognitive function, REM sleep generation and regulation. We suggest that further studies for the cholinergic effects of ChEIs on REM sleep and SDB should be conducted to confirm our conclusion. In our study, follow up scores of ADAS-cog improved, compared to the baseline study. However, a small size of subjects could not generate a statistical significance. In another randomized double blind study, rivastigmine showed increasing ADAS-cog score with improved cognitive function, and participation in activities of daily living in patients with mild to moderately severe AD.222327 Anatomic structures affected by AD, to some degree, overlap with those related to the genesis and control of REM sleep. This made some researchers to speculate that there might be a functional relationship between REM sleep and the pathogenesis of AD.28 The relationship between REM sleep and cognition still requires complex interpretation. The role played by REM sleep disturbances in the cognitive impairment of patients with AD continues to be a promising research field. For clinicians who prescribe drugs to patients with dementia, including probable AD patients, many have considerable comorbidity, including psychiatric or behavioral symptoms, other than sleep disturbance. Among the comorbidity findings, SDB is an important factor that could contribute to cognitive decline in patients with dementia. Clinicians sometimes have enemies on all sides to manage patients with AD and SDB. Fortunately, ChEIs are expected to aid in this issue based on studies that support the positive effects of ChEIs on patients with SDB, including our current study. There are some limitations of small size, short duration of study, and no control group to make a confirmative result from this data. Therefore, further large randomized double-blinded controlled studies are needed to conclude the effect and action of ChEIs on SBD in patients with probable AD.
Figures and Tables
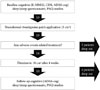 | Fig. 1Study schedule. Nineteen patients enrolled and underwent baseline cognitive and sleep studies. Five patients dropped out due to adverse events related to transdermal treatment. Four patients dropped out for refusal of additional overnight PSG study. Finally, 10 patients were compared, before and after transdermal rivastigmine treatment. ADAS-cog: Alzheimer's disease assessment scale-cognitive, CDR: clinical dementia rating, MMSE: Mini-Mental State Examination, PSG: polysomnographies. |
References
1. Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer's disease. Sleep Med. 2005; 6:347–352.

2. Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004; 6:Suppl 1A. S16–S28.

3. Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol. 2002; 131:135–144.

4. Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010; 74:1416–1423.

5. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984; 34:939–944.

6. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.

7. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991; 14:540–545.

8. Herscovitch J, Broughton R. Sensitivity of the stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981; 4:83–91.

9. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4:561–571.

10. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. Westchester: American Academy of Sleep Medicine;2007.

11. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001; 19:585–591.

12. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984; 141:1356–1364.

13. Gehrman PR, Martin JL, Shochat T, Nolan S, Corey-Bloom J, Ancoli-Israel S. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003; 11:426–433.

14. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342:1378–1384.

15. Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008; 56:2076–2081.

16. Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009; 5:305–309.

17. White DP. Pharmacologic approaches to the treatment of obstructive sleep apnea. Sleep Med Clin. 2016; 11:203–212.

18. Riemann D, Lis S, Fritsch-Montero R, Meier T, Krieger S, Hohagen F, et al. Effect of tetrahydroaminoacridine on sleep in healthy subjects. Biol Psychiatry. 1996; 39:796–802.

19. Moraes W, Poyares D, Sukys-Claudino L, Guilleminault C, Tufik S. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study. Chest. 2008; 133:677–683.

20. Sukys-Claudino L, Moraes W, Guilleminault C, Tufik S, Poyares D. Beneficial effect of donepezil on obstructive sleep apnea: a doubleblind, placebo-controlled clinical trial. Sleep Med. 2012; 13:290–296.

21. Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv. 2008; 5:1377–1386.

22. Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: a review. Int J Clin Pract. 2009; 63:799–805.

23. Han HJ, Lee JJ, Park SA, Park HY, Kim JE, Shim YS, et al. Efficacy and safety of switching from oral cholinesterase inhibitors to the rivastigmine transdermal patch in patients with probable Alzheimer's disease. J Clin Neurol. 2011; 7:137–142.

24. Schredl M, Weber B, Braus D, Gattaz WF, Berger M, Riemann D, et al. The effect of rivastigmine on sleep in elderly healthy subjects. Exp Gerontol. 2000; 35:243–249.

25. Kanbayashi T, Sugiyama T, Aizawa R, Saito Y, Ogawa Y, Kitajima T, et al. Effects of donepezil (Aricept) on the rapid eye movement sleep of normal subjects. Psychiatry Clin Neurosci. 2002; 56:307–308.

26. Schredl M, Weber B, Leins ML, Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp Gerontol. 2001; 36:353–361.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download