Abstract
Background and Purpose
Although subjective cognitive impairment (SCI) is often accompanied by Parkinson's disease (PD) and may predict the development of mild cognitive impairment or dementia, longitudinal brain perfusion changes in PD patients with SCI remain to be elucidated. The current prospective study examined cerebral perfusion changes in PD patients with SCI using technetium-99m hexamethylpropylene amine oxime single photon emission computed tomography (SPECT).
Methods
Among 53 PD patients at baseline, 30 patients were classified into the PD with SCI group and 23 patients were assigned to the PD without SCI group. The mean follow-up interval was 2.3±0.9 years. The Mini-Mental State Examination, Clinical Dementia Rating, and Global Deterioration Scale were used to assess impairments in cognitive function. Brain SPECT images were acquired at baseline and follow-up.
Results
Significant differences between the two groups were not found for demographic variables, PD severity, or cognitive function at either baseline or follow-up. At baseline, the PD with SCI group showed decreased perfusion in the left angular gyrus compared to the PD without SCI group. Longitudinal analysis revealed widespread perfusion reductions primarily in the bilateral temporo-parieto-occipital areas and cerebellum in the PD with SCI group. Relative to the PD without SCI group, an excessive decrease of perfusion was found in the left middle frontal gyrus of the PD with SCI patients.
Parkinson's disease (PD) is widely known as a movement disorder, but, recently, non-motor symptoms of PD such as cognitive difficulties have begun to receive attention.1 A longitudinal study reported that dementia was diagnosed in about 26% of PD patients, and the prevalence of dementia increased to about 80% after 8 years.2 In addition, PD patients have an almost six-fold increased risk of developing dementia compared to the general population.3
Subjective cognitive impairment (SCI) is defined as subjective complaints of cognitive declines with normal levels of cognitive performance on objective measures.4 Several lines of research have shown that SCI may predict the development of mild cognitive impairment (MCI) or dementia in PD patients56 as well as in the healthy elderly.7 Despite the potential of imaging techniques in providing valuable insight for early detection and development of management strategies, only a few in vivo neuroimaging studies have investigated the neural correlations of SCI in PD. Previous magnetic resonance imaging (MRI) studies in PD patients with SCI demonstrated reduced gray matter density in the medial frontal, angular, and anterior cingulate cortex when compared to PD patients without SCI.8 Cortical thinning in the frontal, parietal, and parahippocampal areas were reported in PD patients with SCI compared to healthy controls.9 In addition, a single photon emission computed tomography (SPECT) study found that PD patients with SCI showed hypoperfusion in the frontal, inferior temporal, and anterior cingulate cortices and in the thalamus compared to those without SCI.10 However, since these studies adopted a cross-sectional design, there is a compelling need to elucidate longitudinal brain changes.
The current prospective SPECT study was intended to examine perfusion changes in PD patients with SCI in comparison with those in PD patients without SCI. SPECT with 99mTc-hexamethylpropyleneamine oxime (HMPAO) is widely avai-lable and especially advantageous in detecting subtle cognitive decline related to SCI in PD patients.10 There are an insufficient number of previous studies on this topic to draw a specific hypothesis, but evidence from literature on PD patients with MCI may be useful in anticipating brain perfusion changes specific to the progression of SCI in PD. In neuroimaging studies, the frontal regions of PD patients with MCI consistently showed cortical atrophy,1112 decreased functional connectivity,13 and hypometabolism.14 Furthermore, executive dysfunction is the most common neuropsychological deficit in PD patients with MCI.1516 Finally, the frontal cortex is also consistently implicated in imaging studies of PD patients with SCI.8910 Therefore, we hypothesized that excessive decreases in regional cerebral blood flow (rCBF) would be prominent in the frontal areas of PD patients with SCI as compared to those without SCI at follow-up.
Patients with PD were recruited at Incheon St. Mary's Hospital (Incheon, South Korea). Owing to the lack of general co-nsensus on the diagnostic criteria of SCI, it was defined as self-reported memory complaints in spite of normal cognitive per-formance on formal neuropsychological tests. Patients were classified into the PD with SCI group or the PD without SCI group based on clinical diagnosis by a board-certified neurologist. PD was diagnosed according to the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria for PD. Fluorinated N-3-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (18F-FP-CIT PET) was also used to establish a diagnosis of PD. Exclusion criteria were patients who have had 1) past or present neuropsychiatric disorders including stroke, head trauma, epilepsy, depression, or brain surgery; 2) significant medical comorbidities such as diabetes mellitus, hypertension, or hypercholesterolemia; 3) cerebrovascular lesions on MRI; 4) any other detectable cause of memory deficits; or 5) lifelong memory complaints. Patients who were taking any psychotropic medications were also excluded. Written informed consent was obtained from all study participants and the study protocol was approved by the Research Ethics Committee.
Physical and neurological examinations were performed by a board-certified neurologist. The severity of PD symptoms was assessed with the Hoehn-Yahr Scale.17 The Clinical Dementia Rating (CDR)18 and Global Deterioration Scale (GDS)19 were used to evaluate the overall severity of dementia. Global cognitive function was measured with the Mini-Mental State Examination (MMSE).20
Brain SPECT scans were conducted at baseline and follow-up. All patients were intravenously injected with 1110 MBq of HMPAO in a dark, quiet room. After approximately 40 minutes, perfusion images were acquired with a dual-head gamma camera (NM640; GE Healthcare, Milwaukee, WI, USA) equi-pped with a low-energy, fan-beam collimator. All images were attenuation corrected and reconstructed in a 128×128 matrix with a voxel size of 3.9×3.9×3.9 mm (field of view=240 mm) using filtered back projection.
We used Statistical Parametric Mapping 12 (SPM; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) for image processing and statistical modeling. All images were spatially normalized to the SPM SPECT template (Montreal Neurological Institute, McGill University, Montreal, Canada), resliced with a voxel size of 2×2×2 mm3, and then smoothed with a 16 mm full-width half-maximum isotropic Gaussian kernel.
Differences in continuous demographic or clinical variables were assessed with the independent t-test or Mann-Whitney U test, while the gender difference was evaluated with the chi-square test. A two-tailed p value of less than 0.05 was considered statistically significant. All analyses were conducted with Stata version 13.1 (StataCorp., College Station, TX, USA).
A series of SPM statistical analyses were conducted with age and sex as nuisance covariates. A two-sample t-test was used to investigate differences in rCBF between the two groups at baseline. A relative threshold masking of 0.8 was applied and global counts were normalized to 50 mL/100 g/min with proportional scaling. The statistical threshold was set at an uncorrected p<0.001 at voxel level with an extent threshold of 100 voxels.
A paired t-test was performed to examine perfusion differences between baseline and follow-up in the PD with SCI group. Reference cluster normalization was used since it provides a significant increase of statistical power in studies on neurodegenerative diseases.21 In brief, analysis with default normalization was performed to identify rCBF increases at follow-up using a threshold of t>2.0.22 The mean rCBF value in the significant area was extracted from each image using MarsBaR toolbox version 0.44 (http://marsbar.sourceforge.net/) and used as a scaling factor for the subsequent analysis of rCBF decreases in follow-up images. The statistical threshold was set at an uncorrected p<0.001 at voxel level with an extent threshold of 100 voxels.
A flexible factorial design was used to assess the group-by-time interaction effect. Perfusion increases specific to the PD with SCI group were determined by the contrast of [(PD with SCI at baseline<PD with SCI at follow-up)>(PD without SCI at baseline<PD without SCI at follow-up)], whereas decreases were revealed by the contrast of [(PD with SCI at baseline>PD with SCI at follow-up)>(PD without SCI at baseline>PD without SCI at follow-up)]. Reference cluster normalization was applied and the statistical threshold was set at an uncorrected p<0.005 at voxel level with an extent threshold of 50 voxels.
The demographic and clinical characteristics of the study participants are presented in Table 1. A total of 53 PD patients were recruited at baseline. Among them, 30 patients were classified into the PD with SCI group. At follow-up, 20 PD with SCI patients and 14 PD without SCI patients participated in the study. The mean follow-up interval was 2.3±0.9 years. None of the participants showed significant cognitive decline when assessed with objective measures (MMSE, CDR, and GDS) at baseline and follow-up. In addition, differences between the two groups were not significant for age, sex, duration of PD symptoms, Hoehn-Yahr score, levodopa equivalent dose, MMSE, CDR, or GDS at baseline and follow-up (all p>0.05).
The results from the SPM analysis are demonstrated in Table 2. At baseline, the PD with SCI group showed decreased perfusion in the left angular gyrus (t=4.36, voxel-level p<0.001, cluster size=200 voxels) compared to the PD without SCI group (Fig. 1). In comparison, the PD with SCI group showed widespread reductions in rCBF in the bilateral cerebellum and temporo-parieto-occipital areas including the right middle temporal gyrus, left lateral occipital cortex, and right precuneus at follow-up (Fig. 2). In addition, we identified an excessive perfusion decrease specific to PD patients with SCI in the left middle frontal gyrus compared to PD patients without SCI (t=3.25, voxel-level p=0.001, cluster size=52 voxels) (Fig. 3).
The current study investigated longitudinal changes in cerebral perfusion in PD patients with SCI using HMPAO SPECT. First, we compared the differences between PD patients with SCI and those without SCI at baseline. Then, changes in rCBF in the PD with SCI group were examined at follow-up. Finally, we examined the group-by-time interaction in order to test for a difference in perfusion changes between the two groups.
At baseline, the PD with SCI group showed rCBF decreases in the left angular gyrus when compared with the PD without SCI group. This is in line with a previous PET study that revealed reduced parieto-temporal glucose metabolism among healthy subjects with SCI.23 Moreover, a structural MRI study indicated significant reductions in gray matter density in the angular gyrus among PD patients with SCI compared to those without SCI.8 The angular gyrus has a strong connection with the parahippocampal gyrus24 and is closely involved in attention and memory retrieval.25 Abnormalities in this area may contribute to the subjective feeling of memory decline.
At follow-up, the PD with SCI group demonstrated widespread rCBF reductions in the temporal, parietal, and occipital cortical regions and the cerebellum compared to the baseline measurements. In line with these results, hypoperfusion has been found primarily in the parieto-occipital areas in studies of PD.262728 Additionally, a meta-analysis of PD suggested that cerebellar perfusion may be unchanged or slightly decreased.26 In support of this view, hypoperfusion29 and hypometabolism30 were found in the cerebellum of PD patients. Increasing evidence indicates certain roles for the cerebellum in the path-ophysiology of PD.31 The progressive perfusion decrease in the cerebellum may reflect pathological changes induced by dopaminergic degeneration.31
When the longitudinal perfusion changes of the PD without SCI group were subtracted from those of the PD with SCI group, an excessive decrease in rCBF was found in the middle frontal gyrus in the latter group. Similarly, a previous cross-sectional SPECT study in PD patients with SCI reported reduced rCBF in the medial frontal regions.10 Moreover, PD patients with MCI14 also showed decreased glucose metabo-lism1432 and cortical atrophy1112 in the middle frontal cortex. During the progression to dementia, neuropathological changes generally start in the memory-related hippocampal and entorhinal cortex, spread into the parieto-temporal areas, and finally affect the frontal cortices.3334 However, neurological deficits in the prefrontal regions may occur in the earlier stages of cognitive decline in PD.35 The prefrontal cortex is known to play an important role in various domains of cognitive functioning by interacting with other brain areas including the hippocampus.36 Functional alterations in the prefrontal regions during the course of SCI in PD may account for subjective ne-uropsychological symptoms, such as deficits in memory retrieval, attention, and executive function.
Potential limitations of this study include classification of the patients with SCI based on self-reported subjective cognitive complaints, which was done because diagnostic criteria for SCI is not yet established. Levels of SCI were not assessed on a continuous scale owing to the lack of validated tools and, therefore, correlations between rCBF and symptom severity could not be examined. Secondly, the MMSE may be not sensitive enough to evaluate frontal dysfunction or exclude patients with MCI or dementia. Detailed neuropsychological batteries will be needed in future studies. Thirdly, the follow-up period was too short to observe a progression from SCI to MCI or dementia. Fourth, depressive symptoms were not assessed despite the fact that depression is a major comorbid condition in PD and might have exerted an influence on SCI.37 In addition, cerebral artery stenosis was not evaluated by angiography. Finally, the Hoehn-Yahr score and levodopa equivalent dose were assessed only at baseline. Although PD symptoms were not of primary interest in the current study, detailed descriptions of PD severity such as the Unified Parkinson Disease Rating Scale scores would be helpful to define patient characteristics.
In conclusion, the current longitudinal SPECT study provided insights into rCBF changes in PD patients with SCI and suggested that perfusion deficits in the middle frontal gyrus can be detected in a preclinical stage of both MCI and dementia. Future studies in larger samples using comprehensive neuropsychological test batteries are warranted to investigate whether a perfusion decrease in the prefrontal regions can serve as a reliable and valid biomarker for SCI in PD. In addition, longitudinal comparison of rCBF changes between PD patients with SCI and healthy comparison subjects would be of clinical relevance.
Figures and Tables
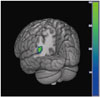 | Fig. 1Decrease in cerebral perfusion in the PD with SCI group compared with the PD without SCI group. The color bar represents voxel-level t-values. PD: Parkinson's disease, SCI: subjective cognitive impairment. |
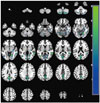 | Fig. 2Decreases in brain perfusion at follow-up in the PD with SCI group compared with baseline. The images are shown in neurological conventions and the color bar represents voxel-level t-values. PD: Parkinson's disease, SCI: subjective cognitive impairment. |
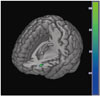 | Fig. 3Excessive decrease in cerebral perfusion specific to the PD with SCI group compared with the PD without SCI group. The color bar represents voxel-level t-values. PD: Parkinson's disease, SCI: subjective cognitive impairment. |
Table 1
Demographic and clinical characteristics of the study participants*

*Data are presented as mean±standard deviation or median (interquartile range), †Independent t-test or Wilcoxon-Mann-Whitney test for continuous variables and chi-square test for sex.
CDR: Clinical Dementia Rating, GDS: Global Deterioration Scale, MMSE: Mini-Mental State Examination, PD: Parkinson's disease, PDSCI: Parkinson's disease with subjective cognitive impairment.
Acknowledgements
This study was also supported by a grant from the Ministry of Science, ICT and Future Planning, Republic of Korea (NRF2015M3C7A1064832).
References
1. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010; 9:1200–1213.

2. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003; 60:387–392.

3. Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology. 2001; 56:730–736.

5. Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol. 2014; 27:276–281.

6. Hong JY, Sunwoo MK, Chung SJ, Ham JH, Lee JE, Sohn YH, et al. Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson's disease. Neurobiol Aging. 2014; 35:1739–1743.

7. Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008; 4:1 Suppl 1. S98–S108.

8. Hong JY, Lee JE, Sohn YH, Lee PH. Neurocognitive and atrophic patterns in Parkinson's disease based on subjective memory complaints. J Neurol. 2012; 259:1706–1712.

9. Hong JY, Yun HJ, Sunwoo MK, Ham JH, Lee JM, Sohn YH, et al. Cognitive and cortical thinning patterns of subjective cognitive decline in patients with and without Parkinson's disease. Parkinsonism Relat Disord. 2014; 20:999–1003.

10. Song IU, Kim JS, Chung SW, Lee KS, Oh JK, Chung YA. Early detection of subjective memory impairment in Parkinson's disease using cerebral perfusion SPECT. Biomed Mater Eng. 2014; 24:3405–3410.

11. Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007; 78:254–259.

12. Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord. 2011; 26:289–296.

13. Amboni M, Tessitore A, Esposito F, Santangelo G, Picillo M, Vitale C, et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J Neurol. 2015; 262:425–434.

14. Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008; 70(16 Pt 2):1470–1477.

15. Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007; 22:1272–1277.

16. Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G;. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009; 72:1121–1126.

18. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39:1159–1165.
19. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean Version of Global Deterioration Scale. J Korean Neurol Assoc. 2002; 20:612–617.
20. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.
21. Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz HG, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer's disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009; 44:43–50.

22. Borghammer P, Aanerud J, Gjedde A. Data-driven intensity normalization of PET group comparison studies is superior to global mean normalization. Neuroimage. 2009; 46:981–988.

23. Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008; 63:609–618.

24. Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006; 16:1418–1430.

25. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013; 19:43–61.
26. Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, Ito K, et al. Cortical hypometabolism and hypoperfusion in Parkinson's disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010; 214:303–317.

27. Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O'Brien JT. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage. 2003; 20:1309–1319.

28. Melzer TR, Watts R, MacAskill MR, Pearson JF, Rüeger S, Pitcher TL, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain. 2011; 134(Pt 3):845–855.

29. Leenders KL, Wolfson L, Gibbs JM, Wise RJ, Causon R, Jones T, et al. The effects of L-DOPA on regional cerebral blood flow and oxygen metabolism in patients with Parkinson's disease. Brain. 1985; 108(Pt 1):171–191.

30. Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson's disease. J Nucl Med. 1996; 37:1115–1122.
32. Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Mov Disord. 2009; 24:854–862.

34. Jeong HS, Park JS, Song IU, Chung YA, Rhie SJ. Changes in cognitive function and brain glucose metabolism in elderly women with subjective memory impairment: a 24-month prospective pilot study. Acta Neurol Scand. 2017; 135:108–114.

35. Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004; 75:1467–1469.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download